Plenary01:A comprehensive theory for the evolution, control and adaptability of avian migration
Peter Berthold
Research Unit for Ornithology of the Max Planck Society, Vogelwarte Radolfzell, Schlossallee 2, Schloss Moeggingen, D-78315 Radolfzell, Germany, fax 0049 7732 150134, e-mail berthold@vowa.ornithol.mpg.de
Berthold, P. 1999. Towards a comprehensive theory for the evolution, control and adaptability of avian migration. In: Adams, N.J. & Slotow, R.H. (eds) Proc.22 Int. Ornithol. Congr., Durban. Ostrich 70 (1): 1 - 11
Theories about the evolution of migratory behaviour in birds have recently been grouped in eight categories (Rappole 1995). Common to all of them is the idea that migration originated in ancestral sedentary populations by some kind of ‘behavioural jump’. These are difficult to explain, especially under the assumption that migration has evolved several or many times independently. Recent experimental studies undertaken to illuminate the genetics of bird migration and the potential and speed of the associated microevolutionary processes have led to another view – a simple yet comprehensive theory. Its central concept is (obligate) partial migration, which is extremely widespread at higher latitudes, possibly also in the tropics, and seems to have evolved very early or might even have been inherited from pre-avian ancestors. Partial migration provides a behavioural turntable from which exclusive migratoriness and sedentariness can easily and rapidly be reached (or left) through selection and related microevolutionary processes. The fact that all important migratory features are directly genetically controlled, that migratoriness and amount of migratory activity are based on a common genetic mechanism, and that migratory syndromes exist, probably all greatly facilitate microevolutionary changes from migratoriness to sedentariness and vice versa.
INTRODUCTION
No one knows how bird migration began, or how it may still be evolving, despite the tremendous theoretical efforts that have been made to explain it (e.g. Gauthreaux 1982). Farner (1955) thought that migratory behaviour may have evolved several or many times in the history of modern birds; if migration thus now represents many cases of divergent and convergent evolution, it could be controlled by various mechanisms. Rappole (1995) recently summarised all the theories related to the evolution of bird migration in eight categories, from the continental drift theory to Baker’s migration threshold model. He wrote, ‘The one factor that all of these theories have in common is an ancestral sedentary population’ and ‘All of the theories begin with something that causes these ancestors to move’. This implies that there was a ‘behavioural jump’ from the sedentary to the migratory condition. Two causes have been proposed: chance mutations and a stepwise development from dispersal through facultative migration to obligate migration, such as Baker (1978) and Terrill (1990) have described. If we accept the postulated polyphyletic origin of bird migration, we have to ask how this trait could have so often occurred independently in different lineages. This question is especially problematic for the considerable number of bird populations that have switched between migratory and sedentary behaviour several times in their history, often rapidly. The Serin Serinus serinus is an example of this flexibility (see below). On the other hand, an evolutionary sequence from dispersal to obligate migration also leaves us with a crucial ‘something’ to explain. As Rappole (1995) put it: ‘Yet, for the phenomenon to be migration, as opposed to dispersal, not only must something force the individual bird away from its point of origin initially, but something must also push it back to its point of origin later in the annual cycle. Also, this something must act over and over again’. Rappole then proposed, ‘What pushes it back is deterioration of weather in the temperate zone’. Yet in obligate migration what triggers departure is not the weather but endogenous factors (e.g. Gwinner 1986; Berthold 1988), and these must find an explanation before a bird migration theory can be made plausible.
I think there is a simple explanation that actually accounts for all phenomena of bird migration that have evolved independently several times or even thousands of times. It is partial migration, or more precisely, a disposition towards partial migratory behaviour, which means the possession of genes for migrating as well as genes for not migrating. A two-track predisposition like this could enable any behaviour within the whole range to be expressed rapidly when needed. A population might thus comprise both migratory and sedentary birds, in whatever proportions, or might be (almost) purely sedentary or purely migratory. As I shall demonstrate, this two-track predisposition appears to be very widespread among birds and may well be the turntable on which the migratory/sedentary behavioural complex rests.
Some introductory examples
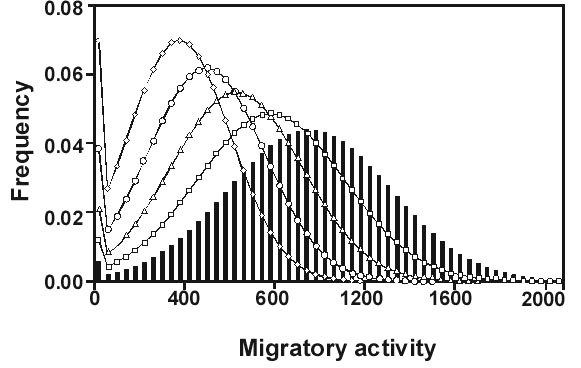
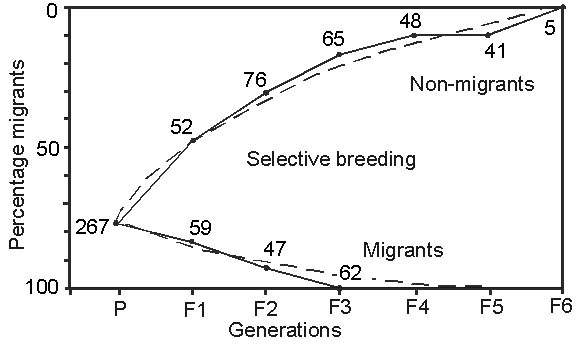
First, consider the results of a crucial experiment that we carried out with Blackcaps Sylvia atricapilla from 1976 to 1989 (Fig. 1). The birds were taken from a partially migratory population in southern France. We hand-raised 267 individuals and showed that in this sample about 75% of the birds were migratory and 25% sedentary, which was in good agreement with the results of field studies. In a subsequent two-way selective breeding experiment in which we bred 458 individuals, we found that genetic factors play an important role in determining whether a bird will be migratory or sedentary. Furthermore, with appropriate selection it took only 3 to 6 generations to make the experimental population completely migratory or almost completely sedentary (Berthold et al. 1990a, Fig. 1).
Evidently there are many bird species that behave in nature like our experimental Blackcaps. One is the Serin, a relative of the canary. This species, the subject of Ernst Mayr’s 1926 dissertation, is mainly a partial migrant. Mayr, for his doctorate, studied the gradual expansion of the Serin’s range since the 19th century, from the Mediterranean region into central Europe. By now the Serin has reached northern Europe (Fig. 2). In central Europe it became a migrant, about which Mayr (1926) made the following remarks (translation): ‘Now, before our very eyes as it were, a development occurred that turned this partial migrant into a migrant. The partial migrant, which in autumn originally wandered off in all directions from its breeding grounds, when it breeds further north becomes a partial migrant that predominantly turns southward, and at our latitude finally becomes a true migratory bird. That even our Serin originally did not migrate precisely towards the south is attested by the fact that in the course of the last century it appeared so often during autumn and winter in northeastern France, Belgium and Holland. It is very remarkable that the migratory drive gradually became stronger and stronger. We can follow this trend best by the occasions on which it was seen to try spending the winter in Germany . . . Winter observations have been reported from almost all parts of the country . . . but with time they are becoming progressively rarer.’ Mayr then asked: ‘Has the migratory drive become stronger by elimination of the individuals with a weak drive (selection) . . . ? . . . I shall leave it to the evolutionary theoreticians to draw conclusions from this behaviour of the Serin! The only thing certain is the fact that the migratory drive has intensified.’
Now, with the course of global warming, the Serin overwinters frequently in all of its central European breeding grounds; that is, it is again a partial migrant, as it used to be in the Mediterranean region (Bauer & Berthold 1997; Berthold 1998). Other bird species may well have regularly behaved like the Serin throughout geological time, oscillating between behaviours that were mainly migratory or mainly sedentary according to the prevailing environmental conditions – for instance, during the sequence of glacial and warm periods. The Blackbird Turdus merula offers a similar example. Today, in the Mediterranean–Atlantic region it is sedentary or a partial migrant. After the postglacial recolonisation of central Europe it was a pure migrant well into the 19th century, then a partial migrant, and now – again probably due to global warming – in some places it is sedentary. In the British Isles, with their mild winters, it has been so for quite some time (e.g. Berthold 1998).
For this kind of behavioural oscillation between migration and sedentariness only two factors are essentially required:
(1) In the population there should be genes both for migrating and for not migrating, which allow rapid microevolution by selection to produce more or less of either form of behaviour and hence give the potential for partial migratory behaviour, and
(2) The genetic bases for controlling the most important parameters of migration should be present, so that selection can operate and microevolution can occur.
The evidence accumulating over the last ten years indicates increasingly that both factors are present, as shall be shown in this paper. I begin by briefly demonstrating that in species in which this question has been examined, all the essential migratory processes and prerequisites are controlled directly by genetic factors, and the same probably applies to many other species. This is followed by a consideration of heritability estimates, microevolutionary potentials and a crucial migration syndrome. After discussing the distribution of the potential for partial migration, I conclude by formulating the new migration theory.
Genetic basis of migratory traits
Regarding the genetic basis, let us reconsider five questions I raised at the International Ornithological Congress in Ottawa in 1986. These were answered at that time by demonstrating the existence of endogenous control factors (Berthold 1988). Since then we have learned from a number of additional crossing and selection experiments that genetic factors in general play a direct role.
Most of the studies have involved our main experimental species, the Blackcap. This is an excellent subject for the investigation of population genetics. It breeds in a vast region extending from the Atlantic Ocean islands in the west to western Siberia in the east and has nonbreeding areas ranging from Great Britain to South Africa. Its populations include migrants, nonmigrants and partial migrants, and the migrants cover long, medium and short distances in various directions, including two migration divides. We have taken experimental birds from ten sites in Europe and Africa (Fig. 3), and hand-raised 2700 individuals, and a total of 1200 were bred in our aviaries.
Since 1993 we have also been working systematically with redstarts genus Phoenicurus. Two species, the Black Redstart Phoenicurus ochruros and the Common Redstart Phoenicurus phoenicurus, interbreed regularly in nature and can also be crossed successfully in captivity (Berthold et al. 1996). In southern Germany the Black Redstart is a short-distance migrant, travelling about 1000 km to Mediterranean winter quarters, whereas the Redstart is a long-distance migrant. Its winter quarters are up to 7000 km away, south of the Sahara. The two species exhibit the classical differences between a short-distance and a long-distance migrant, in seasonal organisation and in migratory disposition. The young hatch at about the same time in May/June, but the Redstart moults sooner and in shorter time, in preparation for its long journey. The Redstart departs early (from August) and travels far, and in the laboratory this is reflected by an early appearance and long duration of the nocturnal migratory restlessness that represents the natural activity. Correspondingly, the Black Redstart’s period of restlessness is late (from October/November) and brief. The Redstart soon becomes much heavier and accumulates large fat depots, whereas in the Black Redstart both processes begin later, are less pronounced and last only a short time (for details see Berthold 1985). These species are therefore ideal for cross-breeding experiments on the inheritance of migratory parameters, migration syndromes etc. Furthermore, F1 hybrids are fertile, and successful back-crossing is known to occur in nature and has also proved possible in captivity. We have now managed to breed about 100 individuals and to produce hybrids and backcrosses (Berthold et al. 1996).
How does a migrant know when to leave?
Earlier studies showed that some species leave higher latitudes so early that summer is still at its peak (Berthold 1996b). These birds are clearly not being driven away by deteriorating environmental conditions; they are induced to depart by endogenous factors, as von Pernau postulated as early as 1702. In more than 15 bird species, this triggering of migration has been found to depend on an endogenous annual periodicity – on circannual rhythms (Gwinner 1986; Berthold 1996a,b). We can now demonstrate that genetic factors are directly involved in the development and termination of migratory activity.
We took Blackcaps from southern Germany, all of which showed intense migratory activity, and crossed them with nonmigratory conspecifics from the Cape Verdes. About 40% of the F1 hybrids developed migratory activity, and the degree of this activity was about intermediate between that of the migratory parents and the (zero) activity of the nonmigratory ones. This tells us, first, that migratory activity or the migration drive can be genetically transmitted into the offspring of a nonmigratory bird population by cross-breeding. And second, the underlying genetic mechanism is a multilocus system rather than a single-locus system (because otherwise all F1-birds would be expected to show some migratory activity; Berthold et al. 1990b). When the birds from southern Germany were crossed with migratory Blackcaps from Russia and nonmigratory Blackcaps from Madeira, the results were consistent with these conclusions (unpubl. data).
With experiments on captive redstarts we can test whether genetic factors determine not only the migration drive but also the precise time when migratory activity sets in. Preliminary results from 21 Black Redstart–Redstart hybrids are shown in Fig. 4. The hybrids are intermediate between the two parents, not only in the duration of their activity but also in its onset. It follows that the time of onset of migratory activity is also genetically determined. This was previously also indicated by comparing departure times in the field with the onset of migratory activity of hand-raised individuals. In 19 songbird species these comparisons gave such a high correlation coefficient (of 0.967) that the endogenous mechanisms triggering migratory restlessness in captivity must also be assumed to be the main initiators of actual migration in the wild (Berthold 1990).
How does an inexperienced migrant know where to fly?
Observational data and the results of three experiments document that a migrant’s choice of direction is also under direct genetic control. The first is the above-mentioned cross-breeding experiment with migratory and nonmigratory Blackcaps from southern Germany and the Cape Verdes, respectively, in which 40% of the F1 hybrids showed genetically transmitted migratory activity. When seven of these birds were tested in orientation cages, five showed significant directional preferences during autumn and spring migration. They preferred a NE–SW axis, which matched the principal axis of migration of the migratory parental German population (Berthold et al. 1990b). Thus not only the migration drive but also orientation behaviour had been genetically transmitted.
Second, Blackcaps from both sides of the central European migration divide were cross-bred: German birds with a southwesterly direction and Austrian birds with a southeasterly direction. F1 hybrids showed intermediate orientation (Fig. 5), again indicating that genetic factors are directly involved in the control of migratory direction (Helbig 1991).
This experiment yielded another important result. The eastern migrants exhibited a pronounced southward shift in their migratory direction from October to November – just as they do in the wild – whereas the western migrants did not change direction, which corresponds to their natural behaviour. The hybrids of a shifting and a nonshifting parent showed a moderate shift. Hence it appears that the magnitude of the shift is also genetically controlled.
Third, within only 30 years some of the central European Blackcaps have adopted a novel, northwestern direction towards the British Isles that is already being inherited (see below).
Fourth, when birds with this novel northwestern direction were cross-bred with those exhibiting southwesterly directions, their offspring demonstrated intermediate directions (Helbig et al. 1994).
How does a migrant know when to end its journey?
For some time experiments have indicated that inexperienced birds, which perform the first migration on their own, will end their journey and locate previously unknown winter quarters at least partially under genetic control. Over 30 species and populations have been shown to possess specific patterns of migratory activity that evidently serve as time programs for migration, according to calculations by various methods (Berthold 1996a,b). By cross-breeding Blackcaps we have demonstrated that these patterns are genetically determined, population-specific characteristics. Hybrids of southern German medium-distance migrants and Blackcaps from the Canaries, which migrate very little, were almost exactly intermediate in this respect (Fig. 6). According to the vector-navigation hypothesis, these hereditary patterns of migratory activity in combination with inherited migration directions transport inexperienced migrants ‘automatically’ to winter quarters never previously visited (Berthold 1996a,b).
However, these Blackcap data leave something to be desired; because the migration routes of the Canary Island birds are unknown, the amount of their migratory activity cannot be interpreted precisely. The redstarts are better suited in this respect. We know the migration distances of both Redstart and Black Redstart to central Africa and the Mediterranean area, respectively (above), and their ratio is about 7:1. When the amounts of migratory activity of the two species were measured, by recording the migratory restlessness of caged hand-raised individuals, we found a similar ratio of about 9:1 (Berthold 1985). The slight difference between these ratios may be due to some diurnal migratory activity of the Black Redstart which remained unrecorded. Twenty-one F1 hybrids so far investigated showed approximately intermediate patterns of migratory activity, which confirms that the activity patterns are under direct genetic control (Berthold et al. 1996).
When does a migrant start to prepare for migration, and what are the signals for starting?
We had previously shown, for Sylvia warblers, first that early-departing long-distance migrants show a comparatively accelerated juvenile development from the egg-stage onwards, and second that the particularly early and rapid juvenile moult is genetically controlled (Berthold 1996a,b). The latter also applies to F1 redstart hybrids; we have found that the time course of their juvenile moult is intermediate between those of the parental populations (Berthold et al. 1996).
Using redstarts, we also obtained a new result, namely that migratory body-mass development and fat deposition are genetically controlled. Comparison of the body-mass curves of Redstarts and Black Redstarts (indicating large and small migratory fat deposition, respectively) with that of their F1 hybrid offspring revealed that the curve of the hybrids is substantially intermediate, and consequently is determined by genes from both parental populations (Berthold et al. 1996).
What makes an individual either migratory or sedentary in a partially migratory population?
Here partial migration means obligate partial migration; in other words, it refers to populations in which each year some of the individuals migrate while others remain in the breeding area. Two main contradictory hypotheses have been proposed about the control of partial migration. One is the ‘genetic’ hypothesis, put forward by Nice (1933), on the basis of her pioneering studies on Song Sparrows Melospiza melodia, and later by Lack (1943/1944). It states that whether an individual will be migratory or sedentary is already determined in the egg by combination of the parental genes. In contrast, the ‘behavioural-constitutional’ hypothesis, proposed by Kalela (1954), Lack (1954) and others, supposes a facultative determination of migratory behaviour depending on population density, resource availability and especially constitution and social rank. It is thought that the weakest and lowest-ranking individuals, being the losers in various confrontations, are driven to migrate by spacing processes. There are other hypotheses that include both genetic and conditional factors, and migratoriness and sedentariness within partially migratory populations can also depend on age and/or sex (for discussion see Kaitala et al. 1993; Berthold 1996a,b; Dingle 1996; Sutherland 1996).
In the two-way selective breeding experiment with partially migratory Blackcaps from southern France, treated above, we have been able to select the birds to 100% migratoriness or to almost 100% sedentariness within a few generations (Fig. 1). These results indicate, first, that both traits – migratoriness and sedentariness – have a strong immediate genetic basis. Second, their heritability estimates are of the order of 0.6, thus both traits are readily changed by selection, which indicates a huge potential for microevolution.
There are two other obligate partial migrants for which the occurrence of migratory and nonmigratory individuals has been shown to be genetically determined: the European Robin Erithacus rubecula in the experiments of Biebach (1983), and the Blackbird in those of Schwabl (1983). Clear indications have been found for four additional species – Song Sparrow, Stonechat Saxicola torquata, Great Crested Grebe Podiceps cristatus and Silvereye Zosterops lateralis – in studies by Nice (1933); Dhondt (1983); Adriaensen et al. (1993) and Chan (1994).
Nice (1933) found that among Song Sparrows, migratory individuals can be the offspring of sedentary parents and nonmigratory individuals the offspring of migratory parents, as we found in our study of partially migratory Blackcaps. Consequently, partial migration in these species is not based on a simple genetic dimorphism; instead, the two traits migratoriness and sedentariness are likely to be polygenic threshold characters. If so, each of these traits would be represented by stronger or weaker genotypes, depending on how many loci are occupied by genes for one or the other trait. The behaviour of individuals with weak genotypes, close to the threshold, could often easily be modified by environmental influences, that is, there could be genotype-environmental interactions. To some degree such birds could develop a conditional strategy, as Adriaensen et al. (1990) have suggested for robins in Belgium. However, since these authors stated that ‘no changes were observed in the migratory behaviour of an individual during the course of its life’, a conditional strategy remains questionable. Possibly, different European Robin subpopulations have different ratios of genetically determined migrants and nonmigrants depending on the varying speed of microevolutionary processes towards increasing sedentariness. Differentiation of subpopulations appears not to be unlikely (as has been shown in Blackcaps and Blackbirds, Berthold 1996a,b) since ‘migratory and resident males tend to use different habitats’ (Adriaensen et al. 1990).
Most probably, many migratory features in addition to those treated above are genetically controlled. One of these certainly is wing length, as has been shown by cross-breeding experiments with Blackcaps from long- and short-winged populations (Fiedler 1997). The same applies to details of wing shape, as the hybridization of redstarts showed. The Black Redstart has a long sixth primary with a notch on the outer web which is absent in the shorter sixth primary of the Redstart. Hybrids are intermediate in both primary length and depth of the notch (Berthold et al. 1996). Other features likely to be genetically controlled are the choice of food, including the frequent seasonal shifts from insectivorous to vegetable diet during the migratory period, and spontaneous habitat selection in staging areas on the basis of ‘Gestalt perception’ (Berthold 1996b).
In conclusion, in the Sylvia and Phoenicurus species that we have investigated, genetic factors are directly involved in all the basic migratory events as well as in the necessary preparations for migration – and there are numerous indications that this also applies to many other species.
How great are the phenotypic and additive genetic variances of migratory traits?
Next, we have to treat a more general aspect: variation of migratory traits and their microevolutionary potential. The variances of migratory traits must be known if we are to understand their microevolutionary potentials and be able to predict changes due to environmental alterations. Relevant data come from the two-way selective breeding experiment with Blackcaps treated above and also from a specially designed study of the migratory activity of southern German Blackcaps. Essentially, all the individuals from Germany are medium-distance migrants. Most migrate to the Mediterranean region, although some travel as far south as the West African coast from Senegal to Nigeria, and others overwinter north of the Mediterranean (Berthold et al. 1990c and unpublished ringing recoveries of the Vogelwarte Radolfzell). The migratory distances thus range from about 700 to some 4500 km – a ratio of 1:6 (Berthold et al. 1990c). We recorded the migratory activity of 280 hand-raised individuals from 69 families of this population, and we also raised another 94 individuals in a breeding experiment with the aim of assessing the amount of additive genetic variation present in this population. In both cases we found the same ratio of about 1:6 between the smallest and largest amounts of migratory activity. We paired individuals with low, medium and high amounts of migratory activity in our aviaries in order to study the variance of activity in the different groups of offspring. Having measured the migratory activity of the 94 young birds we obtained, we calculated heritabilities by parent–offspring regressions and by full-sibling correlations. Heritability estimates by different methods ranged from 0.37 to 0.46 (Berthold & Pulido 1994). This high phenotypic and additive genetic variation of migratory activity in the German Blackcap population could lead to rapid evolutionary changes. According to the vector-navigation hypothesis – as mentioned above – changes in migratory activity would alter the distance covered during the first migration and thus the choice of the wintering area. Fig. 7 shows a model calculation: predicted responses of German Blackcaps (with 900 units of mean migratory activity) to downward selection. Even under moderate selection a population of short-distance migrants like the migratory fraction of the partially migratory Blackcaps in southern France (treated above, with only 350 units of migratory activity, Fig. 7) could evolve from medium-distance migrants within just 10 to 15 generations.
That such rapid microevolution does indeed occur in the wild is demonstrated by the increasing tendency, over the last 30 years, for central European Blackcaps to overwinter in the British Isles instead of the Mediterranean region. When a ringed individual migrated from Austria to the British Isles (Ireland) in 1961, it was assumed to have lost its way (Berthold 1995). But this northwestern migrant was soon followed by hundreds and thousands of other Blackcaps. We tested 40 of the birds that overwintered on the British Isles (in England) in orientation cages in our laboratory and found, unsurprisingly, that their migratory activity was concentrated in the northwestern direction, towards their new winter quarters rather than towards the Mediterranean (Fig. 8). The offspring of these birds, raised in our aviaries, showed an identical northwestern direction, significantly different from the direction of migratory activity exhibited by the ‘normal’ southern German control birds that were tested simultaneously (Fig. 8). According to these results, although it has been only 30 years since central European Blackcaps first began migrating to the British Isles, this new route – about one-third shorter and in a new direction – has already become genetically determined. It follows that microevolution must have taken place in this brief time (Berthold 1995).
In the two-way selective breeding experiment with partially migratory Blackcaps treated above we were able to select for exclusive migratoriness and sedentariness within a few generations. This experiment provided another discovery that has brought us closer to a new theory about bird migration: a correlated response of increasing migratory activity found in three generations of selection for higher frequency of migrants and of decreasing migratory activity observed in four generations of selection for lower proportion of migrants (Fig. 9). In other words, as the migrants become relatively more numerous, their migratory activity also increases, and conversely. A plausible interpretation is that migratoriness and sedentariness, or incidence of migratory activity, as well as amount of migratory activity in migrants are controlled by one and the same genetic system and represent a coherent migration syndrome. Drawing on the migratory activities of 775 Blackcaps from the partially migratory population in southern France, we tested two alternative genetic models concerning the relationship between incidence and amount of activity (Fig. 10). First, the amount of activity could be the continuous variable ‘underlying’ the phenotypic expression of migratory urge. Alternatively, the expression of the two traits – migratoriness and amount of activity – could be controlled by two separate genetic systems. Both the distribution of activities in different cohorts and the inheritance pattern derived from selective breeding indicate that incidence and amount of activity are two aspects of one trait. Individuals without migratory activity – nonmigrants – simply have activity levels at the low end of a continuous distribution, below a threshold, and thus below the limit of phenotypic expression. This finding has profound implications for the understanding of the evolution of migration. Even in a population that is at present exclusively migratory, selection for lower activity (or, according to the vector-navigation hypothesis mentioned above, for shorter migratory distances) would be expected eventually to reach the critical threshold below which the first nonmigrants appear ‘automatically’, with no assistance by other factors such as mutations, assortative mating, etc. Consequently, selection for lower activity would automatically, step by step, produce partial migration (Pulido et al. 1996; Berthold 1999a,b). This interpretation is supported by the facts that short-distance migrants are often also partial migrants, that phenotypically ‘pure’ migratory populations often contain small proportions of nonmigrants while sedentary populations often contain some migrants (below), and that the migratory fractions of partial migrants normally are exclusively short-distance migrants (e.g. Peterson et al. 1993, with few exceptions such as the Cattle Egret Bubulcus ibis; Maddock & Geering 1994).
From the detailed analysis of the quantitative genetics of migratory activity in southern German Blackcaps a model was designed to estimate when the first nonmigrants would appear if selection for lower levels of migratory activity were to act in such an exclusively migratory population (Fig. 11). It shows that when migratory activity is reduced by selecting individuals in the lowest 30% of the range of activity, the proportion of nonmigrants approaches 7% by the fourth generation. By combining this model with the preceding model for the conversion of medium-distance to short-distance migrants, and by including the results of our two-way selective breeding experiment treated above, we find that in case of strictly directional selection an exclusively migratory population could be converted into a predominantly sedentary one in about 25 generations or 40 years.
In summary, full migratoriness or sedentariness can readily be achieved by microevolution from partial migration, and partial migration and sedentariness very likely from full migratoriness. Only genotypically nonmigratory populations might primarily amount to evolutionary dead ends, and they could achieve migratoriness only secondarily by mutations or, more likely, by acquiring migration genes through interbreeding with (neighbouring) migratory populations.
Distribution of migratoriness and partial migration
If most of the present-day bird populations were partially migratory, others exclusively migratory and only relatively few genotypically nonmigratory, it would be easy for them to change between migratory and sedentary behaviour by microevolution with the genetic control mechanisms considered above. And this seems to be what actually happens as the following examples corroborate. Peterson et al. (1993) classified 407 bird species breeding in Europe as follows: 35% are explicitly classed as ‘partial migrant’, 33% as ‘migrant’ or as ‘summer visitor’ (migrants with winter quarters outside Europe) and 12% as ‘resident’. The remaining 20% are qualified as ‘mainly migrant’, ‘mainly summer visitor’, ‘mainly resident’ or ‘partial resident’; i.e. these are also partial migrants, but with a more or less large proportion of migrants or nonmigrants. When these are included, the proportion of partial migrants rises to 55%. In specialized literature we read that of the remaining 12% classed as residents, even typical ‘nonmigrants’, such as the Capercaillie Tetrao urogallus, Partridge Perdix perdix, Crested Tit Parus cristatus or House Sparrow Passer domesticus, either show some migratory populations, or contain small numbers of migratory individuals in so-called sedentary populations (e.g. Grote 1941; Dolnik 1975; Berthold 1996a,b). On the other hand, there are many examples in the literature of the repeated appearance of nonmigratory individuals in practically purely migratory species such as the Swallow Hirundo rustica, House Martin Delichon urbica or Garden Warbler Sylvia borin which are not stragglers (e.g. Zink 1973–1985; Berthold 1996a,b; Elias 1996). In view of all this evidence, it is not unlikely that most of the bird species now breeding in Europe are genotypical partial migrants; of these, most are also quite clearly phenotypical, and the remainder are currently being strongly selected for one phenotype, so that the other appears very rarely.
It is generally taught that the tropical avifaunas consist mainly of sedentary forms. But this opinion is progressively being refuted. Winker et al. (1997) wrote: ‘assumptions of sedentariness in tropical birds should be made with extreme caution’. Rappole (1995) referred to intratropical and subtropical migration in similar terms: ‘migratory movements are much more common among Neotropical residents than has been recognized . . .’, ‘many species of tropical residents are actually migratory to a greater or lesser extent . . .’, ‘species may not disappear completely . . .’, and ‘most of the smaller species would probably be nocturnal migrants’ (implying: perform true migratory flights). And with respect to austral migration he adds: ‘Roughly 75% of the species involved in austral migration have populations that undertake partial, nondisjunct migration . . .’ Similar reports have been presented for Neotropical birds by Levey & Stiles (1992), for southcentral Africa by Dowsett (1988), and for Australia by Chan (pers. comm.) Levey & Stiles (1992) stated: ‘seasonal movements are common in Neotropical forest birds’ and ‘we hypothesize that seasonal movements within the tropics predisposed these birds to migration out of the tropics’. We can conclude that widespread migratoriness is by no means restricted to the higher latitudes; it is also common in tropical regions. Given that many of the intratropical and subtropical migrations occur over short distances and that short-distance migrants are often partial migrants (e.g. Peterson et al. 1993), partial migration is presumably also common there. Thorough investigation, especially of those forms and regions that have previously been neglected, may well make it clear that partial migration is the most widespread mode of life among birds – a basic pattern of avian behaviour. For instance, Newton (1979) wrote about raptors: ‘Nearly every raptor species that has been studied performs some kind of migratory movement in at least part of its range’. There is little doubt that detailed studies will bring to light numerous cases of partial migration.
Partial migration is by no means a bird-specific behaviour; members of many other phyla have ‘invented’ it as well. According to Lundberg (1988) it appears to be a widespread phenomenon among some plant and many animal taxa. Numerous examples are given for plants (e.g. Impatiens capensis, Spergularia spec.), crustacea (e.g. Daphnia galeata, D. hyalina), insects (e.g. Gryllus firmus, Callosobruchus maculatus, Oncopeltus fasciatus, Cicadulina mbila, Melanoplus sanguinipes, Spodoptera exempta), fish (e.g. Gasterosteus aculeatus, Oncorhynchus tshawytscha, Salmo trutta, Coregonus clupeaformis, Oncorhynchus nerka, Osmerus mordax, Salvelinus alpinus), amphibians (e.g. Rana esculenta), reptiles (e.g. Geochelone gigantea) and mammals (e.g. Microtus agrestis and Orcinus orca) (Fairbairn & Roff 1990; Hoffmann 1994, Dingle 1996; Smith & Skulason 1996; Sutherland 1996; Gatehouse 1997 and Spaak & Ringelberg 1997). This brief overview is by no means complete and many other examples may be added. Thus, partial migration is obviously a behavioural strategy that has existed for a long time and is broadly established.
With respect to the geographical area of origin of bird migration, Rappole (1995) convincingly presented the view that, at least ‘for many Nearctic–Neotropical migrants, migration is a behaviour that has developed principally in tropical resident species . . .’. The principal evidence supporting this ‘southern ancestral home theory’, earlier formulated by Cooke (1915), in which migrant ancestors originated in the tropics, ‘is the extensive taxonomic information documenting the existence of close typical resident relatives for most long-distance migrants (78%) to the Nearctic’. Other authors, such as Feduccia (1980) and Johnson & Herter (1990), have suggested that ‘Bird migration may have originated as early as the Eocene, about 50 million years ago, shortly after modern forms of birds first appeared’, and there is also some evidence that the toothed flightless Hesperornis of the Cretaceous period, which lived about 80 million years ago, were already migratory or partially migratory and migrated by swimming (Tyrberg 1986). And Alerstam (1990) stated that ‘bird migration has no doubt existed for as long as birds have been present on earth, for more than 100 million years’.
SYNOPSIS AND THE NOVEL THEORY
Now, when I assemble all the data and plausible inferences treated above in a logical context, in five core statements, I believe we shall have a new, simple and yet comprehensive theory of bird migration:
(1) Birds developed migratory behaviour very early, close to their origin in both space and time.
(2) Like the birds themselves, their migratory behaviour evolved in tropical regions, or at least under tropical/subtropical conditions, long before temperate zones or areas with corresponding conditions were colonized. Therefore migration was initially over short distances. From short-distance migration, partial migration also arose at an early stage.
(3) It is also possible that birds did not even need to develop migratory and dispersal behaviour themselves; they might already have inherited them from pre-avian ancestors, and perhaps also inherited partial migration, which by that time had long been a common phenomenon.
(4) Whether received or newly evolved, in either case partial migration is likely to have become widespread quickly, as an extremely adaptable and successful mode of life. It might even have soon become an evolutionarily stable behavioural mechanism (i.e. some kind of a genetic dimorphism with the two traits migrant and nonmigrant, see also Lundberg 1987, Maynard Smith 1992, Dingle 1996, Sutherland 1996), which to some extent was coupled with a conditional strategy (see above).
(5) Once partial migration had become established, whatever the details may have been, birds possessed a basic behavioural pattern that could be developed in various directions by simple selection and relatively rapid microevolution, so that a whole range of behaviours became available. From partial migrants they could convert to pure migrants and proceed to intercontinental long-distance migration; or they could go the other way, to sedentariness. And most of these developments – up to genotypical pure sedentariness, if it exists – could and can at any time, even now, be reversed, if the environmental conditions require it. Selection in the opposite direction suffices, with no need for a mysterious ‘something’, an obscure ‘behavioural jump’, a mutation or the like.
This theory, then, actually explains everything we know about the categories of bird migration and their changes in time. I think it offers a number of advantages: it is very simple, parsimonious in the best sense of the word; it involves very little speculation; and its most important statements are either based on experimentally confirmed findings or can be tested in the foreseeable future.
Specialists may wonder why I have not drawn a clear distinction between obligate and facultative partial migration, with different control mechanisms. The reason is that I now think they differ more in degree than in principle. A number of facultative partial migrants, such as titmice or waxwings, along with their major, irruptive movements also exhibit a low level of regular partial migration (e.g. Brugger et al. 1994; Berthold 1996a,b; Heldbjerg & Karlsson 1997). It may be that the difference between the two types lies only in the genetic determination of the threshold (Pulido 1998) – and this, too, will soon be testable. In this context it is worth recalling that four decades ago Kalela (1954) suggested ‘an evolutionary connection of true bird migration with some kinds of irregular migration or irruption’, referring to tit movements as mentioned above. Possibly, there is also an evolutionary link between true migration and dispersal. According to Dingle (1996) dispersal should ‘be dropped as a synonym for one-way movement’ and restored to its use as a description of the process leading to a distributional outcome of movement. If dispersal is understood as a programmed one-way migratory movement in which the return has been suppressed or delayed by evolutionary processes (Berthold 1999b), then it may well have originated in a common source of various avian migratory movements. Information (at present almost nonexistent) about the genetics of avian dispersal, in particular, could shed light on such a possible connection.
The theory presented here clearly differs fundamentally from Baker’s (1978) migration threshold model. Rappole (1995) has fittingly characterised the central ideas of Baker’s lengthy approach to develop this model as follows: ‘Baker (1978), in an attempt to formulate a general theory to explain all types of animal migration, proposed that each organism has a genetic ‘migration threshold’. When conditions deteriorate sufficiently at point of origin, the individual migrates . . . The problem with Baker’s hypothesis is that it is so broad that it provides little illumination of the process of evolution of migration in specific instances’. Hence, the crucial difference between the theory presented here and Baker’s model is as follows. According to the novel theory only those individuals in which the overall gene expression of a polygenic constitution, above a critical threshold, results in preprogrammed migratoriness, will migrate, and if this genotype is given, migratoriness will normally, except for some conditional or facultative movements, occur regardless of the environmental conditions. The ratio of migratoriness to sedentariness in a population will change substantially only when selection and evolutionary processes have either produced altered genotypes (with respect to their composition of genes expressing migratoriness or sedentariness) or, perhaps, have altered the threshold (Pulido 1998). In Baker’s (1978) model such a strict genetic control mechanism is only one possibility among others (‘Variation in migration response can also reflect evolved innate individual differences in migration threshold’ etc.).
Rapid evolutionary shifts from (more) migratoriness to (predominant) sedentariness in birds appear to be greatly facilitated by two facts: first, that birds in general, nonmigrants as well as migrants, seem to be equipped with a fundamental capacity to successfully perform migratory movements if required and, second, that certain of the essential migratory features, normally forming functional units, are commonly transmitted as migratory syndromes.
As Baker (1978), Terrill (1990), Berthold (1996a,b) and others have outlined, a number of the orientation mechanisms of birds, such as compass mechanisms and navigation systems, were most likely inherited from pre-avian ancestors rather than newly developed. In fact, mechanisms like the magnetic or sun compass, navigation based on magnetoreception, migratory fat metabolism and fat deposition have been in existence much longer, with their origins probably going back to the Precambrian period, and occurring in bacteria, in invertebrates and in all of vertebrate phyla (e.g. Gibo & McCurdy 1993; Hamner et al. 1994; Kle 1994; Lohmann & Lohmann 1996; Friebe 1997). Lawson (1994), for instance, found little variation in navigational skills between migrating and nonmigrating Garter Snakes (Thamnophis sirtalis and T. ordinoides), and the same appears to be true for birds. Baker (1978) stated ‘It now appears that the navigational mechanisms are virtually identical, at least in principle, whether the animal spends its entire life within a few square metres or whether it wanders over an entire ocean . . . It also resolves the paradox that the ability to navigate was best known, best studied, and equally well marked in a ‘resident’ animal, the homing pigeon’. It is therefore not surprising that orientation capability is also well developed in partially migratory birds (Streif 1997). Munro et al. (1993) suggested ‘that the mechanisms controlling avian migration are similar in all birds irrespective of their systematic relation and the location of their distribution range’. This would be the exact expectation if current avian migration systems are thought to originate in common roots by selection and microevolutionary processes.
In a number of animal taxa, from insects through fish to birds, so-called migratory syndromes have been demonstrated or been shown to be likely (Dingle 1986, 1996; Fairbairn 1994: ‘correlated traits hypothesis’). Often, migration and other important behavioural traits do not occur in isolation, but show genetic correlations with traits such as the life history, morphological, or physiological characters that are expressed in a combined form – syndromes or ‘strategies’ (Dingle 1986). In birds the above-mentioned genetic correlation of the degree of migratoriness and the amount of migratory activity may be the most important syndrome, but others may also exist – for example, correlations between the onset and other parameters of migratory activity or among migratoriness, fat deposition or wing characteristics (Pulido & Berthold 1998; Berthold 1999a). Both the general, fundamental capacity of birds to successfullly perform migratory movements, if a migratory drive comes into play, and the possession of favourably evolved migratory syndromes certainly predispose extant birds for rapid microevolutionary shifts from sedentariness to migratoriness and vice versa, as required. Selection on one trait, for instance, will cause appropriate changes in a set of several other traits as well, and the underlying migratory syndromes will accelerate a bird’s readiness to behave altogether more like a resident or like a migrant.
At present, at least in the northern hemisphere, a great number of bird species are changing their migratory behaviour clearly and quickly, very probably in connection with global warming. For hundreds of species, such as hummingbirds of North America, passerines in central Europe and geese in Siberia, various new developments are being reported: (1) later departure in autumn, (2) earlier arrival in spring, (3) shortening of migratory distances, and (4) reduced migratoriness, indicated by increasing sedentary fractions in partial migrants and a beginning of sedentariness in short-distance migrants (e.g. Berthold & Querner 1995; Hill et al. 1998; for review see Berthold 1998). A fifth category – increasing migratoriness – is extremely rare and is just now being examined in the House Finch Carpodacus mexicanus by Able & Belthoff, in connection with its transplantation from California to northeastern America. According to the new migration theory, most of these changes should be considered as microevolutionary processes, and they – and many others that can be expected – will provide excellent tests of the theory.
This new theory provides an orderly framework for one of the most multifaceted complexes of morphological adaptations, physiological processes and behaviour patterns known to science. If the theory proves to be correct, Albert Einstein’s statement that ‘Most of the fundamental ideas of science are simple in themselves, and as a rule can be expressed in language that everyone can understand’ would now apply to bird migration.
REFERENCES
Adriaensen, F., Dhondt, A.A. & Matthysen, E. 1990. Bird migration. Nature 347: 23.
Adriaensen, F., Ulenaers, P. & Dhondt, A.A. 1993. Ringing recoveries and the increase in numbers of European Great Crested Grebes (Podiceps cristatus). Ardea 81: 59–70.
Alerstam, T. 1990. Bird migration. Cambridge; Cambridge University Press. 420 pp.
Baker, R.R. 1978. The evolutionary ecology of animal migration. London: Hodder and Stoughton: 1012 pp.
Bauer, H.-G. & Berthold, P. 1997. Die Brutvögel Mitteleuropas. Bestand und Gefährdung. Wiesbaden: Aula: 715 pp.
Berthold, P. 1985. Vergleichende Untersuchung von Jugendentwicklung und Zugverhalten bei Garten- und Hausrotschwanz, Phoenicurus phoenicurus und Ph. ochruros. J. Ornithol. 126: 383–392.
Berthold, P. 1988. The control of migration in European warblers. Acta XIX. Congressus Internationalis Ornithologica, Ottawa 1986: 215–249.
Berthold, P. 1990. Wegzugbeginn und Einsetzen der Zugunruhe bei 19 Vogelpopulationen – eine vergleichende Untersuchung. Proceedings of the International 100. DO-G Meeting, Current Topics Avian Biol. Bonn 1988: 217–222.
Berthold, P. 1995. Microevolution of migratory behaviour illustrated by the Blackcap Sylvia atricapilla: 1993 Witherby Lecture. Bird Study 42: 89–100.
Berthold, P. 1996a. Control of bird migration. London: Chapman & Hall: 355 pp.
Berthold, P. 1996b. Vogelzug. Eine kurze, aktuelle Gesamtübersicht. Darmstadt; Wissenschaftliche Buchgesellschaft: 252 pp.
Berthold, P. 1998. Vogelwelt und Klima: gegenwärtige Veränderungen. Naturwiss. Rundschau 51: 337–346.
Berthold, P. 1999a. Bird migration: genetic programs with high adaptability. DZG Review, Zoology 101, in press.
Berthold, P. 1999b. Vogelzug: eine aktuelle Gesamtübersicht. 4. Aufl.; Darmstadt; Wiss. Buchges., in press.
Berthold, P., Helbig, A., Mohr, G., Pulido, F. & Querner, U. 1996. Aktueller Forschungsschwerpunkt: Vogelzug – moderne Phänomenologie und experimentelle Analyse der Steuerungssysteme und Evolutionsvorgänge. Jb. Max-Planck-Ges. 346–354.
Berthold, P., Helbig, A.J., Mohr, G. & Querner, U. 1992. Rapid microevolution of migratory behaviour in a wild bird species. Nature 360: 668–669.
Berthold, P., Mohr, G. & Querner, U. 1990a. Steuerung und potentielle Evolutionsgeschwindigkeit des obligaten Teilzieherverhaltens: Ergebnisse eines Zweiweg-Selektionsexperiments mit der Mönchsgrasmücke (Sylvia atricapilla). J. Ornithol. 131: 33–45.
Berthold, P. & Pulido, F. 1994. Heritability of migratory activity in a natural bird population. Proc. Roy. Soc. Lond. – B. Biol. Sci. 257: 311–315.
Berthold, P. & Querner, U. 1981. Genetic basis of migratory behavior in European warblers. Science 212: 77–79.
Berthold, P. & Querner, U. 1995. Microevolutionary aspects of bird migration based on experimental results. Israel J. Zool. 41: 377–385.
Berthold, P., Querner, U. & Schlenker, R. 1990c. Die Mönchsgrasmücke. Neue Brehm-Bücherei Nr. 603. Wittenberg Lutherstadt: Ziemsen: 180 pp.
Berthold, P., Wiltschko, W., Miltenberger, H. &. Querner, U. 1990b. Genetic transmission of migratory behavior into a nonmigratory bird population. Experientia 46: 107–108.
Biebach, H. 1983. Genetic determination of partial migration in the European Robin (Erithacus rubecula). Auk 100: 601–606.
Brugger, K.E., Arkin, L.N. & Gramlich, J.M. 1994. Migration patterns of Cedar Waxwings in the eastern United States. J. Field Ornithol. 65: 381–387.
Burton, J.F. 1995. Birds and climate change. London: Christopher Helm, A. & C. Black: 376 pp.
Chan, K. 1994. Nocturnal activity of caged resident and migrant silvereyes (Zosteropidae: Aves). Ethology 96: 313–321.
Cooke, W.W. 1915. Bird migration. Washington, D.C.; U.S. Department of Agriculture Bulletin 185.
Dhondt, A.A. 1983. Variations in the number of overwintering Stonechats possibly caused by natural selection. Ring. & Migr. 4: 155–158.
Dingle, H. 1986. Evolution and genetics of insect migration. In: Danthanarayana, W. (ed.) Insect flight: dispersal and migration. Berlin: Springer-Verlag: 11–26.
Dingle, H. 1996. Migration. The biology of life on the move. New York, Oxford: Oxford University Press: 474 pp.
Dolnik, V.R. 1975. Migracionnoe sostojanie Ptic. Moskau: Academia Nauk USSR: 400 pp.
Dowsett, R.J. 1988. Intra-African migrant birds in south-central Africa. Acta XIX. Congr. Int. Ornithol., Ottawa 1986: 778–790.
Elias, G. 1996. Ocorrência invernal de Andorinha-dos-beirais Delichon urbica em Portugal Continental. Airo 7: 80–85.
Fairbairn, D.J. 1994. Wing dimorphism and the migratory syndrome: correlated traits for migratory tendency in wing dimorphic insects. Res. Pop. Ecol. (Kyoto) 36: 157–163.
Fairbairn, D.J. & Roff, D.A. 1990. Genetic correlations among traits determining migratory tendency in the Sand Cricket, Gryllus firmus. Evol. 44: 1787–1795.
Farner, D.S. 1955 The annual stimulus for migration. Experimental and physiologic aspects. In: Wolfson, A. (ed.) Recent Studies of Avian Biology, Urbana; University of Illinois Press: 198–237.
Feduccia, A. 1980. The age of birds. Cambridge (Mass.): Harvard University Press: 196 pp.
Fiedler, W. 1997. Der Flugapparat der Mönchsgrasmücke (Sylvia atricapilla): Erfassungsmethoden, intraspezifische Variabilität, phäno- und genotypische Varianz, Selektionsgründe und ökophysiologische Bedeutung. PhD Thesis, University of Tübingen, Tübingen, Germany.
Friebe, R. 1997. Orientierung von Meeresschildkröten im Erdmagnetfeld. Naturwiss. Rundschau 50: 24–25.
Gatehouse, A.G. 1997. Behavior and ecological genetics of wind-borne migration by insects. Ann. Rev. Entomol. 42: 475–502.
Gauthreaux, S.A. 1982. The ecology and evolution of avian migration systems. In: Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian biology. Vol. 6; New York; Academic Press: 93–168.
Gibo, D.L. & McCurdy, J.A. 1993. Lipid accumulation by migrating monarch butterflies (Danaus plexippus L.). Can. J. Zool. 71: 76–82.
Grote, H. 1941. Ueber Zugerscheinungen bei sogenannten Standvögeln. Schr. Physik.-ökon. Ges. Königsberg 72: 119–129.
Gwinner, E. 1986. Circannual rhythms. Berlin: Springer: 154 pp.
Hamner, W.M., Hamner, P.P. & Strand, S.W. 1994. Sun-compass migration by Aurelia aurita (Scyphozoa): population retention and reproduction in Saanich Inlet, British Columbia. Mar. Biol. 119: 347–356.
Helbig, A.J. 1991. Inheritance of migratory direction in a bird species: a cross-breeding experiment with SE- and SW-migrating Blackcaps (Sylvia atricapilla). Behav. Ecol. and Sociobiol. 28: 9–12.
Helbig, A.J., Berthold, P., Mohr, G. & Querner, U. 1994. Inheritance of a novel migratory direction in central European Blackcaps. Naturwissenschaften 81: 184–186.
Heldbjerg, H. & Karlsson, L. 1997. Autumn migration of Blue Tit Parus caeruleus at Falsterbo, Sweden 1980–94: population changes, migration patterns and recovery analysis. Ornis Svecica 7: 149–167.
Hill, G.E., Sargent, R.R. & Sargent, M.B. 1998. Recent change in the winter distribution of Rufous Hummingbirds. Auk 115: 240–245.
Hoffmann, A.A. 1994. Behaviour genetics and evolution. In: Slater, P.J.B. & Halliday, T.R. (eds) Behavioural evolution. Cambridge: Cambridge University Press: 7– 42.
Johnson, S.R. & Herter, D.R. 1990. Bird migration in the Arctic: a review. In: Gwinner, E. (ed.) Bird migration. Berlin: Springer: 22–43.
Kaitala, A., Kaitala, V. & Lundberg, P. 1993. A theory of partial migration. Amer. Nat. 142: 59–81.
Kalela, O. 1954. Populationsökologische Gesichtspunkte zur Entstehung des Vogelzuges. Ann. Zool. Soc. Zool. Bot. Fennicæ Vanamo 16: 1–30.
Kle. 1994. Phylogenetische Diversität magnetotaktischer Bakterien. Naturwiss. Rundschau 47: 21.
Lack, D. 1943/1944. The problem of partial migration. Brit. Birds 37: 122–30, 143–150.
Lack, D. 1954. The natural regulation of animal numbers. Oxford: Clarendon Press: 343 pp.
Lawson, P.A. 1994. Orientation abilities and mechanisms in nonmigratory populations of Garter Snakes (Thamnophis sirtalis and T. ordinoides). Copeia 2: 263–274.
Levey, D.J. & Stiles, F.G. 1992. Evolutionary precursors of long-distance migration: resource availability and movement patterns in neotropical landbirds. Amer. Nat. 140: 447–476.
Lohmann, K.J. & Lohmann, C.M.F. 1996. Detection of magnetic field intensity by sea turtles. Nature 380: 59–61.
Lundberg, P. 1987. Partial bird migration and evolutionarily stable strategies. J. Theor. Biol. 125: 315–360.
Lundberg, P. 1988. The evolution of partial migration in birds. Trends Ecol. Evol. 3: 172–175.
Maddock, M. & Geering, D. 1994. Range expansion and migration of the Cattle Egret. Ostrich 65: 191–203.
Maynard Smith, J. 1992. Evolutionsgenetik. Stuttgart: Georg Thieme: 288 pp.
Mayr, E. 1926. Die Ausbreitung des Girlitz (Serinus canaria serinus L.). J. Ornithol. 74: 571–671.
Munro, U., Wiltschko, R. & Wiltschko, W. 1993. A comparison of migratory orientation of birds living in the northern and the southern hemisphere. Ring 16: 5–15.
Newton, I. 1979. Population ecology of raptors. Berkhamsted: T. & A.D. Poyser: 399 pp.
Nice, M.M. 1933. Zur Naturgeschichte des Singammers. J. Ornithol. 81: 552–595.
Peterson, R.T., Mountfort, G. & Hollom, P.A.D. 1993. Collins field guide. Birds of Britain and Europe. London: Harper Collins Publishers: 320 pp.
Pulido, F. 1998. Evolutionary quantitative genetics of migratory restlessness in the Blackcap (Sylvia atricapilla). PhD Thesis, University of Konstanz, Konstanz, Germany.
Pulido, F. & Berthold, P. 1998. The evolution of migratory behaviour in the Blackcap: effects of genetic variances and covariances on evolutionary trajectories. Fauna Selvatica 102, in press.
Pulido, F., Berthold, P. & van Noordwijk, A.J. 1996. Frequency of migrants and migratory activity are genetically correlated in a bird population: evolutionary implications. Proc. Nat. Acad. Sci. USA 93: 14642–14647.
Rappole, J.H. 1995. The ecology of migrant birds. Washington: Smithsonian Institution Press: 269 pp.
Schwabl, H. 1983. Ausprägung und Bedeutung des Teilzugverhaltens einer südwestdeutschen Population der Amsel Turdus merula. J. Ornithol. 124: 101–116.
Smith, T.B. & Skulason, S. 1996. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Ann. Rev. Ecol. Syst. 27: 111–133.
Spaak, P. & Ringelberg, J. 1997. Differential behaviour and shifts in genotype composition during the beginning of a seasonal period of diel vertical migration. Hydrobiologia 360: 177–185.
Streif, M. 1997. Zugorientierung und Heimkehrverhalten der Mönchsgrasmücke (Sylvia atricapilla). PhD Thesis, University of Konstanz, Konstanz, Germany.
Sutherland, W.J. 1996. From individual behaviour to population ecology. Oxford: Oxford University Press: 213 pp.
Terrill, S.B. 1990. Ecophysiological aspects of movements by migrants in the wintering quarters. In: Gwinner, E. (ed.) Bird migration: Physiology and Ecophysiology. Berlin, Heidelberg, New York; Springer: 130–143.
Tyrberg T. 1986. Cretaceous birds – a short review of the first half of avian history. Verh. Ornithol. Ges. Bayern 24: 249–275.
Winker, K., Escalante, P., Rappole, J.H., Ramos, M.A., Oehlenschlager, R.J. & Warner, D.W. 1997. Periodic migration and lowland forest refugia in a ‘sedentary’ neotropical bird, Wetmore’s Bush-Tanager. Conserv. Biol. 11: 692–697.
Zink, G. 1973–1985. Der Zug europäischer Singvögel. Möggingen: Vogelzug: 4 Lieferungen.
Fig. 1. Results of a two-way selective breeding experiment with partially migratory Blackcaps Sylvia atricapilla (from the Mediterranean area). Nonmigrants were bred up to the F6-generation and migrants up to the F3-generation. Numbers indicate individuals tested in each generation. The broken lines represent mathematical functions that best fit the selection response (Berthold et al. 1990a).

Fig. 2. The Serin’s Serinus serinus northward expansion of its breeding range in Europe from 1800 (Burton 1995).
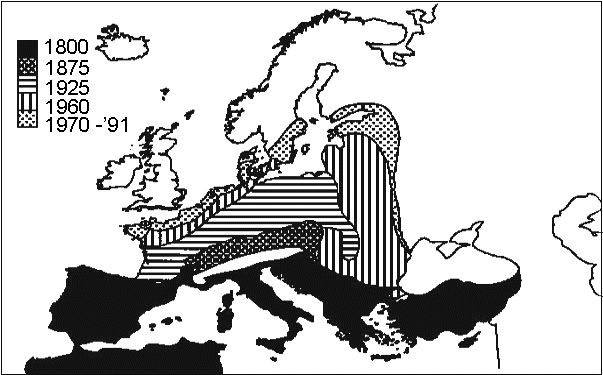
Fig. 3. Schematic representation of the life-forms and migration routes of the Blackcap Sylvia atricapilla. Broken line: border of breeding range, shaded: wintering areas, M: exclusively migratory population, P: partially migratory, N: nonmigratory, arrows: main routes of migration away from breeding grounds, dots: places where experimental birds were collected.
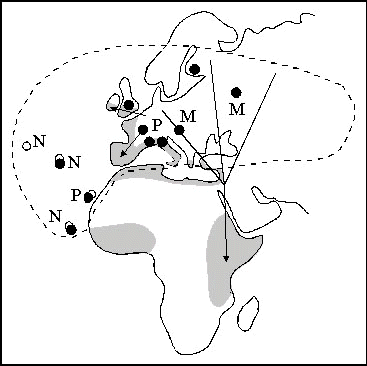
Fig. 4. Mean values and standard errors of time course of migratory activity in hand-raised Redstarts Phoenicurus phoenicurus (lower white bar, n = 12), Black Redstarts Phoenicurus ochruros (upper white bar, n = 12), and of their hybrids (black bar, n = 21). 0: date of hatching of the experimental groups (Berthold & Querner 1995).
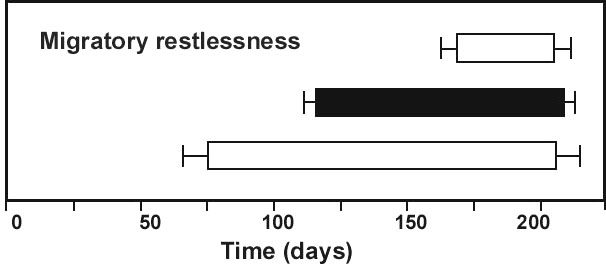
Fig. 5. Individual means of directional choices, and overall means (arrows), of Blackcaps Sylvia atricapilla from Germany (solid symbols) and Austria (open symbols). Inner circle shows data from parents, outer circle from F1-hybrids. Above: data in October, below: November (Helbig 1991).
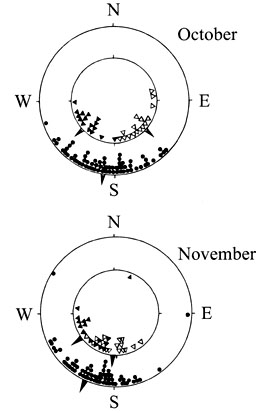
Fig. 6. Time course of migratory activity in groups of hand-raised Blackcaps Sylvia atricapilla during the first migratory period, for birds from southern Germany (SG), Canary Islands, Africa (CI), and their hybrids. Vertical lines: standard error (Berthold & Querner 1981).
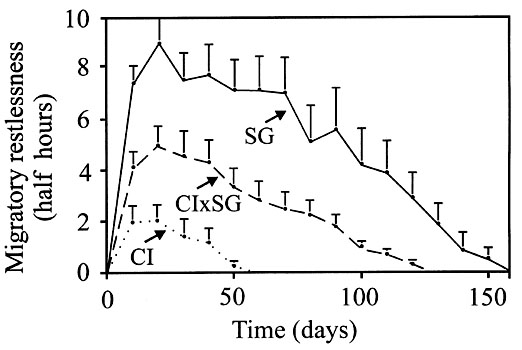
Fig. 7. Predicted responses of southern German Blackcaps Sylvia atricapilla to downward selection on migratory activity (given in half-hours of nocturnal activity), assuming a heritability of 0.41 and 3 different selection regimes. Selection intensities are indicated by the proportions of birds selected per generation (50, 70 and 90%). The broken line gives the average amount of migratory activity in a population of short-distance migrants from southern France (from Berthold & Pulido 1994).
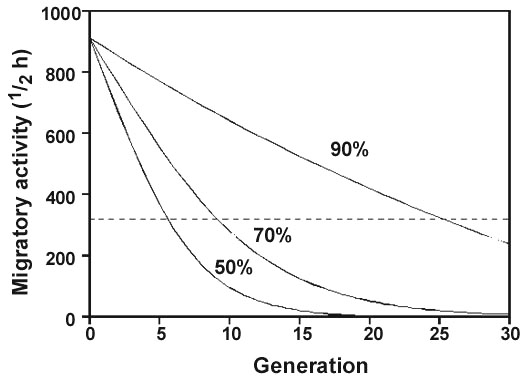
Fig. 8. Orientation of Blackcaps Sylvia atricapilla caught in winter in Britain and tested the following autumn (‘Adults’), of their F1 offspring (‘F1’) and of a control group of hand-raised birds from southwestern Germany. Each symbol gives the mean direction of one bird during 15–20 tests. Among the adults, triangles identify birds that are parents of F1 offspring in upper right diagram. Arrows: group mean vectors (Berthold et al. 1992).
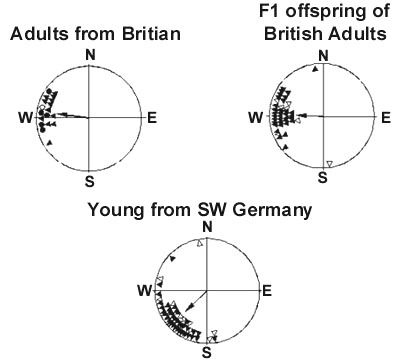
Fig. 9. Correlated response of migratory activity to four generations of directional selection for lower proportion of migrants and three generations of directional selection for higher frequency of migrants. Correlated cumulative selection responses (y-axis) and selection intensities (x-axis) are given in standard deviations of the population mean of the respective parental generation. Points give single-generation selection responses and intensities added to the sum of the selection responses and intensities of the preceding generations. A linear regression line was fitted to these single-generation cumulative selection responses and intensities. The slope of this regression line (b = 0.472) yields a genetic corrrelation of 1 (Pulido et al. 1996).
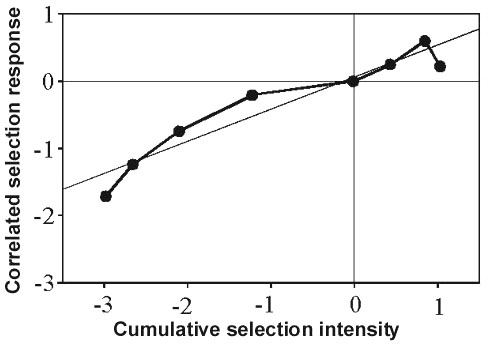
Fig. 10. Models describing the relationship between incidence and amount of migratory activity: In the ‘one-trait-model’ (A), nonmigrants (black bars) are individuals on the left tail of a continuous distribution, with activities below a threshold value T. In the ‘two-trait model’ (B), nonmigrants are randomly distributed over all activity classes; their activities are switched off by genes uncorrelated with the genes controlling the expression of migratory activity (Pulido et al. 1996).
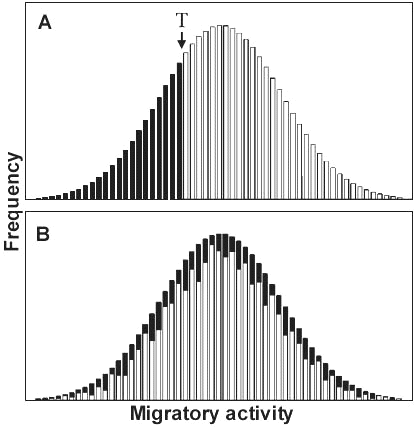
Fig. 11. Predicted change of the distribution of migratory activity and frequency of nonmigrants in the southern German population of Blackcaps Sylvia atricapilla in response to selection for lower activity. The model is based on the actual mean and variance of migratory activity in the southern German population, assuming a heritability of 0.4 and a selection intensity of 1.16 (Pulido 1998).