Plenary02: Energetics and survival of birds in extreme environments
Alexander V. Andreev
Institute of Biological Problems of the North, Russian Academy of Sciences, Portovaya 18, Magadan, 685010 Russia, e-mail
ted_andreev@mail.ruAndreev, A.V. 1999. Energetics and survival of birds in extreme environments.. In: Adams, N.J. & Slotow, R.H. (eds) Proc.22 Int. Ornithol. Congr., Durban. Ostrich 70 (1): 13–22.
As northern climates changed towards those existing at present, bird taxa kept evolving forms adapted to the variety of high-latitude habitats – inland, coastal and alpine. These habitats are structurally simple and dominated by dramatic seasonality and permafrost. These adaptations are expressed in several seasonal and regional avifaunas which include the 20–25 resident bird species of the Siberian taiga, and some 15–45 typical summer visitors to hypoarctic, subalpine and tundra habitats. The survival strategies of birds in these extreme habitats are discussed in the light of experimental data and observations gathered during the seemingly most stressful periods of the birds’ annual life cycle, namely mid-winter in north-eastern Asia and incubation on permafrost in upland tundra. Of the subarctic residents, only grouse utilise predictable browse foods, but have to cope with its low energy content. Winter survival in other taxa depends on utilising fall harvests and individual energy stores. In all cases, the energy utilisation in wintering birds appears to be minimised. In summer, non-resident birds treat their local resources in quite a different way with no evidence of direct energy limitation on reproduction, including incubation. There is little evidence of individual cost-minimisation strategies. Instead, local population-resource balances are regulated through a variety of social mechanisms.
INTRODUCTION
The definition of the term ‘survival’ depends on the selected time-scale as well as on the structural level considered. In this report I will discuss the factors influencing ‘individual seasonal survival’, in both the non-reproductive and the reproductive season, that ensure the production of offspring.
As the geology and landscapes of the northern continents evolved towards their present state, their climates became increasingly colder, and the area of extreme habitats expanded. This long-term trend is presently reflected in the distribution of seasonal temperatures in the Northern Hemisphere (Fig. 1). In the regions of northern Asia, never strongly glaciated, but heavily influenced by quaternary climatic changes, cold-dominated and permafrost-influenced habitats occupy vast areas within the limits of northern taiga and southern tundra (60–67°N ). The range of these habitats that are predictably severe in winter, and highly variable in summer, includes rocky highland deserts, alpine and Arctic tundra, hypoarctic upland and wetland shrubs, polar shoreline areas, and sparse Larch forests.
Following this development, the bird taxa, whose annual life cycle includes several energy demanding episodes, evolved into a number of forms, adapted to using these habitats. These energy demanding episodes include:
wintering in northern taiga and bush-tundra by resident species;
establishing territories and social structures by early spring to ensure clutch formation and incubation needs;
incubation on permafrost;
brooding and growth under unstable weather conditions and restricted or fluctuating food resources of high Arctic and highland tundra habitats.
Of this list, I will discuss only two examples, namely the winter life of northern grouse and the incubation of Arctic birds, chiefly waders.
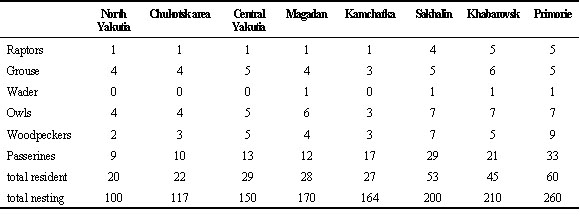
A total of 100–170 bird species occur in, climatically, the most severe regions of northern and central Yakutia, Chukotsk and Magadan area of which 20–28 species, or 16–20%, are year-round residents, capable of surviving the winter lasting 8–9 months with the temperatures predictably below –40°C during 10–12 weeks. Further to the south, the number of nesting species increases markedly, but the proportion of resident species keeps relatively constant (Fig. 2). Resident species (Table 1) include a number of dendrophilic forms belonging to the families of grouse (Tetraonidae), woodpeckers (Picidae), chickadees (Paridae), finches (Fringillidae), and crows (Corvidae), that form the core component of the widespread boreal avifauna (Stegmann 1963).
Two general questions need answering to explain the avian diversity under the challenging winter conditions of northern Asia:
how large are the energy demands that the birds have to tolerate?
how do they meet these to guarantee individual survival?
To cope with the cold, snow, and light-deficient environment birds must find sufficient food on a daily basis and a suitable microhabitat that provides a desirable insulative layer. The list of available winter diets in northern taiga is fairly restricted. The Larch Larix cajanderii, willows Salix sp. Chosenia arbutifolia, birches Betula sp., and alder Alnus sp. provide browse for grouse. The Larch buds or brachyblasts provide food for the Pine Grosbeak Pinicola enucleator; and south of 57°N, the Spruce Picea ajanensis is an important food resource for the Sharp-winged Grouse Falcipennis falcipennis. Seeds of Larch are important for the Redpoll Acantis hornemanni (which also harvests seeds of the Dwarf Birch Betula middendorfii), the White-Winged Crossbill Loxia leucoptera, and the chickadees Parus cinctus and P. montanus. Seeds of the Shrub Pine Pinus pumila provide the chief diet for the Nutcracker Nucifraga caryocatacthes, which stores food underground before the onset of winter and associated snowfalls, and the Nuthatch Sitta europaea, which stores a considerable amount of food under tree bark.
Arboreal invertebrates ensure winter survival of the woodpeckers Picoides tridactylus, Dryocopus martius and favour winter survival of the chickadees, the Nuthatch and the Siberian Jay Perisoreus infaustus, which is also known to establish arboreal stores of a diverse range of foods for winter. Small rodents, small passerines and grouse provide the main, but unpredictable, food source for wintering owls (Nyctea scandiaca, Strix nebulosa, Surnia ulula, Aegolius funereus).
For many months, vast areas of the interior of north-east Asia are the domain of killing frosts. Nonetheless, there exist naturally several warmer microclimates, which may provide some relief in the life of wintering birds or become a necessary condition for their survival.
Snow cover and permafrost
As the air temperature drops below –20°C by mid-October, and the snow cover protects the ground, the permafrost with a year-round temperature maintained at near –12°C, becomes a locally warmer microhabitat. The snow layer is used occasionally by finches and owls for roosting; sometimes, chickadees enter snow tunnels under curved tree trunks to roost overnight (Sulkava 1969; Novikov 1972; Andreev 1980). However, using the snow cover is not typical behaviour for wintering passerines and owls, presumably due to the loose structure of their plumage. However, the plumage of grouse is of the required density and roosting in snow burrows is a typical feature of their behaviour and indeed a basic requirement for their winter survival;
River valleys and river ice
The floors of the greater river valleys of north-east Asia are formed by loose sediments, soaked with filtering water. These also provide a locally warmer microclimate during many of the winter months. Redpolls were observed to combine warming up and roosting on the bare ice patches, when air temperature dropped below –45°C. At the same air temperature, the chickadees can be seen to roost in the daytime on the bare gravel spots near driftwood logs (Andreev 1980).
Thermal inversions
In montane areas cooler air moving down from higher altitudes forms a thermal gradient up to +3°C per 100 m altitude. Thus, ascending in the timberline zone (approx. 900–1100 m in the Kolyma Upland) one would experience a microclimate 15–25°C warmer than that of the foothills and valleys beneath. Indeed, many chickadees, nuthatches, redpolls and ravens tend to dwell in the timberline zone during the winter.
Solar radiation
On clear days in the areas around the Arctic circle, birds tend to spend the hours around noon on the treetops, gathering some warmth from weak, but apparently important, solar radiation.
Hence, among wintering birds only grouse have diets and thermal shelters that are predictably stable and readily available. Other wintering birds have to rely either on the fluctuating food resources (seeds, insects, rodents) and/or expending effort on food storage before the onset of winter.
WINTER SURVIVAL OF PALEARCTIC GROUSE
In the following analysis I compare energetics and survival of two Palearctic grouse species, one typically boreal (the Sharp-winged Grouse, Falcipennis falcipennis) and the other typically subarctic (the Willow Grouse, Lagopus lagopus). They are of similar body size (600–700 g) and their winter biology affords proper eco-energetic measurements and comparisons.
The principal winter diet of the Sharp-winged Grouse is evergreen Ajan spruce leaves (needles). The bird’s distribution is restricted by the occurrence of this tree; both ranges tightly coincide, being centred around lower reaches of the Amur River (52°N). Winters in that area are comparatively mild (January average –26°C), snowy (about 60 cm by mid-winter) and short (120–130 days, November through March).
The largest in the family, the distribution range of the Willow Grouse is spread across both Eurasia and North America. Optimal conditions, however, occur on the hypoarctic plains and foothills of north-east Asia, north-west Canada and Alaska in the vicinity of 68–70°N, where tall willows form a distinctive vegetation belt. Willow twigs and buds form the bulk of this species’ winter diet. Winters here last over seven months (October through May), midwinter days are dim and short (4–5 hr) in January, daily temperatures drop below –40°C, and snow is 30 cm thick (snow-burrow limit) by mid-November.
The microclimate of wintering grouse were studied experimentally in caged birds (Volkov 1968; Andreev 1977, 1991a; Korhonen 1980; Marjakangas et al. 1983). Most recently, by means of telemetry, I monitored the plumage surface temperature (PST) of a free-living Sharp-winged Grouse (Fig. 3). Even under large fluctuations in ambient temperatures (–25 to –35°C) the bird experienced a distinctly higher PST than ambient temperature. An example of 24 hr continuous measurement (Fig. 4) explains the background to this phenomenon. Due to snow burrowing, the PST increased to nearly zero at night, the difference between the burrow and the air temperature reached 35°C, and the gradient in the snow roof (6–8 cm wide) varied between 3 and 5°C cm–1. In daytime, when the bird ascended to the tree tops, the PST again increased due to warming by solar radiation. Only at the time of burrowing in and out of the snow do both air temperatures and PST coincide. Unlike all other Palearctic grouse, the Sharp-winged Grouse never enters snow in the daytime and in addition, prefers to sleep in the canopy when night temperatures are warmer than –15°C.
Thermal conditions in the snow burrows of Willow Grouse were measured in a series of aviary experiments, where hand-raised birds were taught to roost in fresh snow (Fig. 5). In these experiments, temperature sensors were used in parallel with the heat flow disks (Andreev 1986a, 1991a). Again, the difference between inside and outside temperatures reached 35–42°C, and the gradient in the roof varied between 4 and 5°C cm–1. The heat density varied significantly, depending on the outward flow direction. According to these measurements, most heat flux occurs through the burrow bottom area, thus explaining the functional importance of the dense ‘snowshoe’ feathering of grouse’s feet. When outer air temperatures exceed –30°C, the inside burrow temperature may reach zero, and, to avoid overheating, grouse make a small ventilation opening in the roof – a simple and efficient solution. Thus, with the snow cover sufficient to construct roosting chambers, the northern grouse are able to create their own ‘thermal blankets’, spending a good proportion of winter time (60–90%) under thermoneutral conditions.
Typically, northern grouse suffer during the early continental winter, when the night temperatures drop well below –30°C, and thin snow cover prevents them from roosting in borrows. To cope with these hardships, the Black-billed Capercaillie, Tetrao parvirostris, which needs a 35–40 cm snow layer, lays down a conspicuous amount of subcutaneous fat reserves (Egorov et al. 1954, Klaus et al. 1989). A similar response was found for the Willow Grouse and the Hazel Grouse, Tetrastes bonasia, in the Omolon valley, Kolyma drainage, 67°N (Andreev 1980). The fat reserves are used typically by December, when snow cover becomes thick enough to roost inside snow burrows. Populations inhabiting milder climates do not lay down much fat reserves. However in the case of Willow Grouse at the Novosibirsk Islands (74–75°N) and especially the Spitsbergen Rock Ptarmigan, Lagopus mutus (77–79°N ) this fall fattening has developed into clear overwintering strategy (Kistchinski 1974; Mortensen 1985). High densities of Snowshoe Hares, Lepis timidus, in the Oimyakon area, north-east Yakutia (62°N) cause frequent disturbance to night-roosting Willow Grouse, which may cause birds to freeze to death at temperatures below –50°C (Isaev 1994). Despite large fluctuations in ambient temperature, all grouse species avoid hypothermia (Andreev 1980).
After the evening feed, during which a grouse fills its crop with a bulky mass of frozen food of 16–22% of the body weight, it enters a snow burrow. The process of digestion in a roosting grouse is strikingly steady, with both the input from the crop (trimmed browse diet) and the output (woody droppings) being highly correlated with time since entering the roost (Fig. 6 & Fig. 7 ). This relationship was first established by Potapov (1974), and used to estimate daily energy budgets in several species of wintering Tetraonidae (Potapov & Andreev 1985). The difference between the gross energy intake, GEI (the energy of food in the crop) and the excretory energy, EE (the energy content of droppings collected from the snow burrow) along with the roosting period, Tn (time spent in burrows estimated through direct observation) gives the required measure, the night metabolism (NM):
NM = (GEI–EE) Tn–1Corrected for the daily activity pattern, this basic parameter can be transformed into another basic measure, the daily energy expenditure (DEE):
DEE = NM*Tn + NM*K1*Tr + NM*K2*Ta + cdt
Where K1 and K2 represent coefficients of the day-time resting metabolism and the activity metabolism. (Dol’nik 1982, 1995), Tr and Ta are the time budget components during the day, and cdt is the thermoregulatory cost (Andreev 1980).
The first measure (NM) was further developed into a correlated series of eco-energetic parameters suitable for intra- and inter-species comparisons, including their seasonal dynamics (Andreev 1982, 1988). In the needle-eating species, this analysis can be performed without hunting the birds (Andreev & Linden 1994; Andreev 1990a).
Data for the Willow Grouse gathered at several sites in north-east Asia (Fig. 8) reveal that NM measured in the wild under a variety of climatic conditions is relatively stable, exceeding the basal metabolic rate (BMR), determined in a metabolic chamber, by only some 10%. The same is true for the DEE, which fluctuates around 1.5 BMR, but with wider variations. If birds were unable to use the ‘thermal blanket’ provided by snow burrows, thus correlating their individual activity-roosting time budget with the ambient temperature, their daily energy expenditure would increase to 3.0 BMR at –50°C, as prescribed by the Scholander model. Although sustainable for short periods by well-nourished birds under experimental conditions (West 1972), this level of energy expenditure could not be maintained on natural diets for longer time periods (Andreev 1992).
Other Palearctic grouse species tested in the wild indicate similar values. The NM slightly exceeds the BMR, and DEE rate is maintained generally below 2.0 BMR (Fig. 9; Andreev 1988). Such values should be expected, because birds are within their thermal neutral zone whilst in the snow burrows, and the only additional heat needed to be generated is for the initial warming of the air inside the burrow and warming the mass of frozen food in the crop. On the other hand, some heat is recouped by the depositing of droppings within the burrow.
The moderate level of the daily energy requirements of 1.5–1.9 BMR in different species gives the opportunity of either shortening the foraging time and being more selective or remaining more cautious during feeding (Andreev 1991b; Swenson et al. 1995). At the same time, a DEE rate near 2.0 BMR seems to be close to the upper limit that the grouse digestive system can efficiently support. The fact that the majority of grouse species so far studied lose body weight during winter, supports this idea. The more severe become the outside conditions, the clearer this relationship (Andreev 1982) (Fig. 10). For further insight, the seasonal DEE trends of the two grouse species – the boreal Sharp-winged Grouse and the subarctic Willow Grouse – are quantitatively simulated (Fig. 11).
As mentioned above, the Sharp-winged Grouse winters under relatively mild conditions. Air temperatures drop to –15 to –35°C with little or no wind, and there are many clear days, as long as 8.5 hours in December. The standard diet of the Sharp-winged Grouse consists of 80 g (dry weight) of Ajan spruce needles per day (Andreev 1990a). The birds have plenty of food (over 400 trees per ha), constant access to the grit, and their body weight remains steady or declines slightly during the course of winter.
The Willow Grouse encounters quite different winter conditions. At 69°N, the mid-winter temperatures drop to between –35 and –50°C, there are frequent winds and snow storms, and dim light days last less than 5 h a day in December. The Willow Grouse’s daily food consumption includes 90–110 g (dry weight) of willow twigs, buds and bark. The availability of willow browse strongly depends on the snow conditions, its quality continuously worsens as winter progresses, and in many cases, the birds cannot find the grit in subarctic winter habitats. Their body weight declines continuously throughout the winter.
With all these differences, both species consume about 1600 kJ.d–1 by mid-winter. This represents similar calculated DEE rates of 435–450 kJ.d–1, implying DEE = 1.5 BMR. Similar DEE levels occur in March (440–480 kJ.d–1), but to keep up with this demand, the Willow Grouse has to consume much more food (2200 kJ.d–1, representing about 60 g extra fresh food consumption per day). This is indicated by the excretory energy output. Marked differences in dropping mass were found in different Willow Grouse populations, the trend being to increase further to the north (Fig. 12).
These changes reflect the continuous worsening of the quality of the winter food of Willow Grouse. This is manifested as both a simplification of the diet and a deterioration in its nutritive value (Andreev 1992). As winter progresses, snowfalls force the birds to move in the valleys, concentrating in the strips of tall willows. Thereafter, the fine willow and dwarf birch shoots, a typical diet in October and November, get completely replaced by thicker willow twigs (December–February), and in March–April the diet includes a substantial proportion of plain willow bark (Fig. 13). In parallel, the proportion of protein in the diet declines, whereas fibre content increases (Fig. 14).
Worthy of mention is that, when birds are free to choose their foods, as in cage experiments or during late summer in the wild, the diet consists of approx. 16% protein and some 15% fibre. Such a diet provides good digestibility (40–45%) and supports steady or slightly increasing body weight. Natural winter diets, however, differ considerably from preferred diets. When fibre content exceeds 25%, and that of protein drops below 10%, some additional body protein is required to meet daily energy demands. This can only be restored by the consumption of better quality foods. By early winter or under warmer conditions, the feeding birds are highly selective for the finest parts of willow bushes. In the cage experiments, even a slight increase of dietary protein markedly improves its overall digestibility and stabilises body weight (Andreev 1992). However, by mid-winter selective feeding becomes stressful; the quantity of grits declines, resulted in lower food digestibility, approximately 0.20 in March–April against 0.32–0.34 in October (Andreev 1982). Hence, the Willow Grouse has to pass through a nutritional bottleneck, which keeps narrowing until snow starts to melt.
To cope with all these changes, imposed by the stressful environmental conditions, the birds have to eat progressively more food, extracting the required amount of energy, but losing their body mass (Andreev 1992). Other northern grouse have to cope with similar limitations, though less dramatically expressed (Andreev 1988).
To summarise, the combination of the availability of browse and snow cover, characteristic of northern landscapes, creates a niche that allows sustainable over-wintering. Boreal grouse can survive a winter day without relying on high quality diets or even snow burrowing. Surviving a winter month means the bird has to optimise its daily energy budget, chiefly through reducing the heat losses in the snow by using it as a ‘thermal blanket’. Surviving the entire winter involves a trade-off between the energy demands on one side and the food selectivity, diet quality, and body mass resources on the other side. This trade-off is a part of the survival strategy for any grouse (Fig. 15). and uses the benefit of the warm season to overcome the costs of the cold season. The northernmost species, genera Lagopus, evolved to widen these limits and thrives in the subarctic landscapes.
ENERGY COST OF INCUBATION IN ARCTIC BIRDS
Breeding birds in the Siberian Arctic (70°N) tend to synchronise their hatching period with the peak in yearly temperatures during the last week of June and early July. This is followed by the peaks of vegetation growth and invertebrate activity, making tundra habitat a 24 h heaven for growing chicks. To be so timed, many Arctic non-passerine breeders have to start their breeding season by late May–early June, when the environment is still hostile and energy demanding. Incubation in flat Arctic habitats is a stressful period for several reasons. Below the clutch, the surface of the permafrost acts as a heat sink, drawing heat out of eggs and forcing birds to generate more heat. Occasional disturbance by tundra predators makes parents leave the clutch to distract the predator. Individuals of species that incubate alone have to leave their nests periodically to feed. The clutches must be warmed on the adult’s return. Cooling causes little or no harm to the embryos but places additional energy costs on the parent at a time when the bird is already stretched to balance its energy budget. Therefore, it makes the incubation a stressful period, and raises the question ‘how much energy do birds in Arctic environments have to invest in developing embryos?’
To answer this question one has to evaluate the Daily Productive Energy of incubation (DPEi), which is a component of the daily energy expenditure (Fig. 16). To measure the DPEi a number of indirect approaches have been developed (Dolnik 1995). I have contributed to this field by suggesting measurement of the specific heat flows both in clutches and nest cups (Andreev 1984a). The idea was based on the obvious distinction between the eggs placed in an incubator and natural clutches developing under pulsed heat flows generated by the parental brood patch. By the direct measurement of these flows, one could add a key parameter into a description of the entire process, and thus obtain a measure of parental investment in the embryonic development (Andreev 1986a,b, 1994). I call this parameter the ‘central heat flow’ (qc) determined as the specific power flow, generated by the parental brood patch, passing through the eggs and measured in the geometrical centre of the nest cup right below the clutch. To measure the qc, I applied semiconductor heat flow sensors (Andreev 1984a), which produce recordings similar to those of temperature probes, but the output parameter refers directly to power units (Fig. 17). Through several empirical coefficients (Ki), the qc can be related to the heat flows in the clutch mid-section (qe), which being multiplied by the clutch cross-section (Se) gives the required parameter – the cost of steady state incubation:
(Qi): Qi = qc*Ki*Se .
In many northern birds, the cost of incubation is higher than that of birds breeding further south, but still remains reasonably low. For example the DPEi varies from 0.08 BMR (Anserini) to 0.15–0.4 (Anatini) and 0.7 BMR (Tetraonidae) (Andreev 1986b). The comparison of incubation parameters between the Arctic Willow Grouse and the boreal Sharp-winged Grouse (Table 2) reveals that heat losses in the former species are twice as high, and the total amount of energy invested in the clutch is almost three times higher. However, as a proportion of BMR, even Willow Grouse appear far less energetically stressed than Arctic waders.
The waders, comprising about 20% of any local Arctic checklist, represent the extreme example of handling of these environmental conditions and are chosen as an example to answer the questions posed earlier. Their clutch mass varies from about 30% to over 100% of the female’s body mass. The evolutionary tendencies – one of maximising egg size and another of having bigger clutches – coincide in waders. In many cases in Arctic waders this results in the production of four cone shaped eggs, which completely fill the nest hollow.
Measurements have demonstrated clearly that temperatures in the nests of Arctic waders are fairly stable, despite highly variable environmental conditions (Kondratyev 1982; Andreev 1994). Therefore, to maintain constant clutch temperatures, heat flows must show compensatory changes. As the clutch temperature varies slightly around 30°C, the central heat flow varies from 6.5 to 12.5 mW. cm–2, (average flow 10.5 mW. cm–2) (Fig. 18). Being derived from the incubating bird’s metabolism, central heat flow correlates with the body mass (Fig. 19) and increases in a stepwise fashion through the course of incubation. This presumably reflects the embryonic heat production as well as the parental prehatching stress (West & Norton 1975). Power input in watts for the steady state incubation (Qi) depends on the body weight in grams (Wb) following the allometric equation:
Qi = 129 Wb0.29 (Andreev 1994).Among waders weighing 25–100 g, this would vary from 0.5 to 0.95 BMR.
Clutches incubated by a single parent are temporarily abandoned for foraging or in response to disturbance. When exposed, the clutch immediately begins to cool with a hyperbolic decline in temperature and heat flow. The rewarming process lasts about 1.5 times longer than the preceding cooling. With single incubators, the colder the weather, the shorter are exposure periods; when two parents incubate, this relationship does not exist (Kondratyev 1982; Andreev 1994).
The instant rewarming input in watts (Qh) for single parents is found to depend non-linearly on the body mass, although at a faster rate than previously described:
Qh = 39.7Wb0.79For birds weighing 25–100 g this would vary from 1.3 to 1.4 BMR, providing the clutch cooling does not exceed 5°C. In paired species, the Qh becomes negligible. This result explains well why single incubating waders are so sensitive to the ambient temperature during exposure (Andreev 1994).
The proportions of the energy expenditure allocated to different components of energy budget look different, reflecting the daily time budgets of paired and non-paired species. To make this clear, I simulated the DEE for a 50 g bird, assuming that nest attentiveness in a non-paired species equals 0.87, and in a paired species that the cost of incubation is shared equally (Fig. 20). Hence, at 10°C both species would expend about 154 kJ bird–1day or 3.3 BMR (calculated lower critical temperature 28°C). In this case the DPEi would comprise about 11% in the paired species and some 23% in the non-paired species. When expressed as a proportion of basal metabolic rate, the cost of incubation is 0.37 and 0.78 BMR respectively.
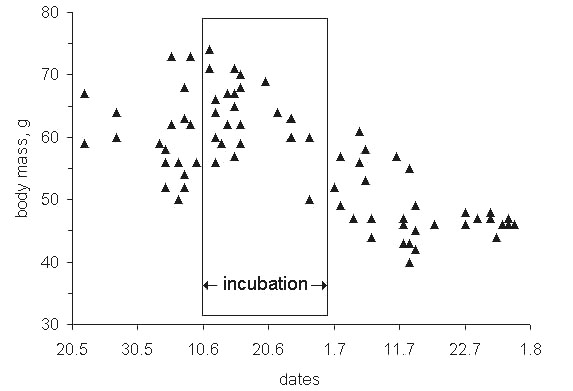
Like many other Arctic breeders, incubating waders constantly lose body mass. When both parents incubate, each one loses less than single nesters (Norton 1973). For example, a Pectoral Sandpiper Calidris melanotos female weighing on average 68 g loses about 1% body weight daily (Fig. 21). This accounts for approximately 19.5 kJ of the expected 150 kJ.–1 or 13% DEE (0.5DPEi). The female must meet its energy shortfall mostly from resources in the adjacent habitat. This is even more so for the paired species (Andreev 1989).Hence, to incubate successfully in the Arctic environment, waders must maintain fairly high metabolic rates (approx. 3.0 BMR and over) and exploit their local food resources intensively. In paired species much of the DEE is spent acquiring food and for territorial activity, whereas in the single bird incubators a greater proportion of the DEE is channelled into the clutch. What would be the biological sense of single-bird incubation in tundra habitats? All species which have been studied in detail (Calidris melanotos, C. temminckii, Philomachus pugnax) demonstrate that their territorial and social relations operate to decrease the population load on the habitat resources during incubation, resulting in an increase in nesting density, which provides the incubating parent with a reliable food surplus and increases the entire population breeding success (Andreev 1989; Khlebosolov 1990).
CONCLUSION
Extreme habitats have a simple biotic structure and low productivity. Many birds, which make extensive use of these habitats demonstrate high levels of adaptability and frequently appear as thriving populations. A substantial proportion of the local species’ assemblage are year-round residents. What is known about their energetics in the Siberian winter indicates the prevalence of the minimisation strategy. Despite the highly demanding conditions, the wintering birds are able to maintain the cost of everyday life between 1.5 and 2.0 BMR (Andreev 1980). Derived from the rough estimates for the Tetraonidae and the Passeriformes, this conclusion is now well justified only for northern grouse. The biological purpose of the minimisation strategy seems to be ultimately shortening foraging time under extremely low temperatures. In this way unacceptable heat losses can be reduced, which would otherwise rapidly put individual energy budgets into a downward spiral (Andreev 1979). Territorial behaviour seems to be of minor importance under winter conditions, and the social organisation serves to minimise both the feeding time and the risk of predation.
In contrast, during the reproductive season, individual energy expenditures of northern birds are somewhat higher. The tendency to such maximisation is expressed in Arctic waders, who can maintain DEE of 2.8–4.0 BMR over the course of eight or nine weeks, including two and half weeks of a special phenomenon – incubation on permafrost (Andreev 1984b, 1989, 1990b; Gavrilov 1988). These expenditure levels are not outstandingly high among birds. So what is surprising is that Arctic birds, the waders especially, do not utilise obvious efforts to keep energy costs low. Instead, they have evolved a variety of territorial and social structures, which, through different ways allow them to increase the population reproductive output. The biological purpose of the apparently ‘wasteful’ practices of Arctic waders seem directed to be towards stabilising population parameters. The population sizes of less conspicuous species tend to be more variable (Andreev 1989).
In all physiologically stressful seasons, the accumulation of internal body resources are a part of survival strategy, involved in the long-term tradeoff between available food resources and individual diet requirements. In both wintering and incubating birds they help birds to pass through bottleneck periods, balancing the unstable triad of foraging time, energy demands, and energy expenditures.
REFERENCES
Andreev, A.V. 1977. Temperature conditions in snow burrows of the Hazel Grouse (Tetrastes bonasia kolymensis). Ecologia 5: 93–95. (In Russian)
Andreev, A.V. 1979. On the relation between the energy balance and the time budget in the ecology of northern birds. Doklady Akademii Nauk SSSR (Reports of the Academy of Sciences of the USSR), 246, 3: 756–759. (In Russian)
Andreev, A.V. 1980. Avian adaptation to subarctic winter conditions. Nauka, Moscow: 174 pp. (In Russian)
Andreev, A.V. 1982. Winter energetics and time budgets in fluctuating populations of ptarmigan, Lagopus lagopus (L.), in northeast Asia. In: Dolnik, V.R. (ed.) Time and energy budgets in free-living birds. Leningrad, Nauka: 68–69. (In Russian)
Andreev, A.V. 1984a. Experimental study of energetical parameters of incubation in Arctic birds. Doklady Akademii Nauk SSSR (Reports of the Academy of Sciences of the USSR), 278, 5: 1269–1273. (In Russian)
Andreev, A.V. 1984b. Individual energetics and species’ adaptive characters in tundra birds. In: Adaptation of organisms to the conditions of the Far North. Tallin: 15–20.
Andreev, A.V. 1986a. Instrumental methods in the studies of ecological energetics of birds. In: Experimental methods in the studies of northern birds and the results of their application. Vladivostok: 59–83. (In Russian)
Andreev, A.V. 1986b. Nest heat flows and incubation energetics in tundra birds. In: Experimental methods in the studies of northern birds and the results of their application. Vladivostok: 84–107. (In Russian)
Andreev, A.V. 1988. Ecological energetics of Palearctic Tetraonidae in relation to chemical composition and digestibility of their winter diets. Can. J. Zool. 66, 6: 1382–1388.
Andreev, A.V. 1989. Ecological energetics and adaptive strategies in northern birds. D.Sc. Thesis, Leningrad. (In Russian)
Andreev, A.V. 1990a. The winter biology of Siberian Spruce Grouse (Falcipennis falcipennis) in the Priamurie. Zoologichesky Zhurnal, 69, 3: 69–80. (In Russian)
Andreev, A.V. 1990b. Ecological energetics of the Arctic chicks. Contemporary ornithology, Moscow, Nauka: 5–21. (In Russian)
Andreev, A.V. 1991a. Winter adaptation in the Willow Ptarmigan. Arctic, 44, 2: 106–114.
Andreev, A.V. 1991b. Winter habitat segregation in the sexually dimorphic Black-billed Capercaillie Tetrao urogalloides. Ornis Scandinavica 22: 287–291.
Andreev, A.V. 1992. The role of fiber and proteins in ecological energetics of the Willow Grouse in winter period. Ecologia, 2 : 56–67. (In Russian)
Andreev, A.V. 1994. Energy cost of incubation in the Arctic waders. Russian Ornithological Journal, 3(4) : 291–302. (In Russian)
Andreev, A.V.& Linden, H. 1994. Winter energetics of the capercaillie – a methodological approach. Ornis Fennica, 71: 33–42.
Dolnik, V.R. 1982. Methods of study of time and energy budgets in birds. In: Dolnik, V.R. ed. Time and energy budgets in free-living birds. Leningrad, Nauka : 3–36. (In Russian)
Dolnik, V.R. 1995. Resources of energy and time of birds in the wild. Proc. Zool. Inst., 179, St Petersburg, Nauka, 360 pp. (In Russian)
Egorov, O.V., Labutin Yu.V. & Mezhenny, A.A. 1954. Data on the biology of the Black-billed Capercaillie in Yakutia. Proc. Institute of Biology , Siberian Department, Soviet Academy of Sciences, 6: 97–105. (In Russian)
Handbook of the climates of the USSR. 1966. v. 33–34, Hydrometeoizdat, Leningrad, 1966. (In Russian)
Gavrilov, V.V. 1988. Territorial distribution, diurnal activity, time and energy budget in Wood Sandpiper males Tringa glareola (Charadriidae) during prenesting period. Zoologichesky Zhurnal, 67, 10: 1549–1559. (In Russian)
Isaev, A.P. 1994. The grouse birds of the Central Verkhoyansk region. Ph. D. Thesis. Petrozavodsk (In Russian)
Khlebosolov, E.I. 1990.Trophic relations and social organization of birds. Vladivostok: 124 pp. (In Russian)
Kistchinski, A.A. 1968. Birds of the Kolyma Upland. Moscow, Nauka, 187 pp. (In Russian)
Kistchinski, A.A. 1974. On the ecology of the Willow Grouse on the Novosibirsk Islands. Ornitologia, 11: 198–205. (In Russian)
Klaus, S., Andreev, A.V. Bergmann, H.-H., Mueller, F., Porkert, J. & Wiesner, J. 1989. Die Auerhuhner, Wittenberg Lutherschtadt: Ziemsen, 288 pp.
Kondratyev, A.Y. 1982. Biology of waders in the tundra of north-east Asia. Moscow, Nauka: 182 pp. (In Russian)
Korhonen, K. 1980. Microclimate in the snow burrows of Willow Grouse (Lagopus lagopus). Annales Zoolgi Fennici 17, 1: 5–10.
Krechmar, A.V., Andreev A.V. & Kondratyev, A.Y. 1978. Ecology and distribution of birds in the north-east USSR. Moscow; Nauka: 195 pp. (In Russian)
Krechmar, A.V., Andreev A.V. & Kondratyev, A.Y. 1991. The birds of northern plains. Leningrad; Nauka: 288 pp. (In Russian)
Lobkov, E.G. 1986. Nesting birds of Kamchatka. Vladivostok: 291 pp. (In Russian)
Marjakangas , A, Rintamjaki, H. & Hissa, R. 1983. Thermal responses in the Black Grouse roosting in burrows. Suomen Riista 30: 64–70.
Mortensen, A. 1985. Survival of the fattest. Ph.D. Thesis, University of Tromse, Tromso, Norway.
Nechaev, V.A. 1992 Birds of Sakhalin Island. Vladivostok, 744 pp. (In Russian)
Norton D.W. 1973. Ecological energetics of calidrine sandpipers at Barrow, Alaska. Ph.D. thesis, Univ. of Alaska, Fairbanks.
Novikov G.A. 1972. The use of under-snow refuges among small birds of the sparrow family. Aqula, Ser. Zool. 13: 95–97. (In Russian)
Potapov, R.l. 1974. Adaptation of the family Tetraonidae to winter season. In: Studies on the biology of birds. Leningrad; Nauka: 207–251. (In Russian)
Potapov, R.L. & Andreev, A.V. 1985. Time and energy budgets in wintering Tetraonidae. Acta XVIII Congressus Internationalis Ornitologici, v. 1, Moscow; Nauka: 409–412.
Stegmann, B.K. 1963. The problem of the Beringian continental land connection in the light of ornithogeography. In: Pacific basin biogeography: Symp. 10th Pacific Sc. Congr. Pacific Sc. Assoc., Honolulu, Hawaii, 21 Aug.–6 Sep. 1961, Ed. J.L. Gressit. Bishop Museum Press: 65–78.
Sulkava, S. 1969. On small birds spending the night in the snow. Aquila, Ser. Zool., 7: 33–37.
Swenson, J.E., Andreev, A.V. & Drovetski, S.V. 1995. Factors shaping winter social organization in Hazel Grouse Bonasa bonasia: a comparative study in the eastern and western Palearctic. J. Avian Biol. 26: 4–12.
Volkov, N.I. 1968. Experimental study of the temperature conditions in the snow burrows of the grouse birds. Zoologichesky Zhurnal, 47, 2: 283–286. (In Russian)
Vorob’ev, K.A. 1954. The birds of Ussuriland, Moscow, 357 pp. (In Russian)
Vorob’ev, K.A. 1963. The birds of Yakutia. Moscow, 336 pp. (In Russian)
West, G.C. 1972. Seasonal differences in resting metabolic rate of Alaskan ptarmigan. Comp. Bichem. Physiol. 42A: 867–876.
West, G.C. & Norton, D.W. 1975. Metabolic adaptations of tundra birds. In: Vernberg, F.J. (ed.) Physiological adaptations to the environment. Intext Educational Publ. N.Y.: 301–329.
Table 1. Composition of the assemblage of wintering birds in eight regions of north-east Asia.

Sources: Krechmar et al. 1978, 1991; Lobkov, 1986; Nechaev 1992; Vorob’ev 1954, 1963.
Table 2. Comparison of incubation parameters in two grouse species.
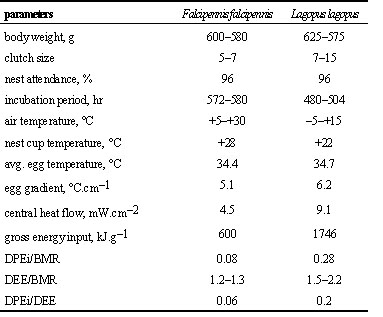
Fig. 1. Distribution of sum of the average daily temperatures in the northern hemisphere during the cold season: 0°C and below (based on data from the Handbook of climates 1966).
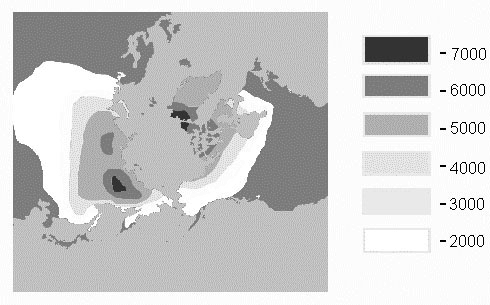
Fig. 2. Number of year-round resident and migrating birds in eight regions of north-east Asia based on original data and from Krechmar et al. 1991 (N.Yakutia, Chukotsk area), Vorob’ev 1963 (Central Yakutia), Kistchinski 1968 (Magadan), Lobkov 1986 (Kamchatka), Nechaev 1992 (Sakhalin, Khabarovsk), Vorob’ev 1954 (Primorie).
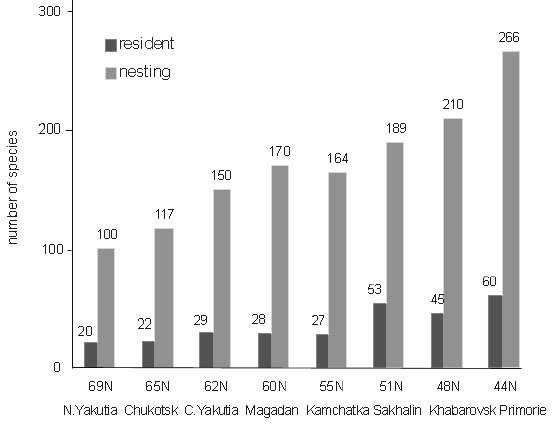
Fig. 3. Plumage surface temperatures (n) of wintering Sharp-winged Grouse (female, 650 g) against ambient temperature (–) and telemetry measurements at the Lower Amur, 52°N, in the second half of January 1997.
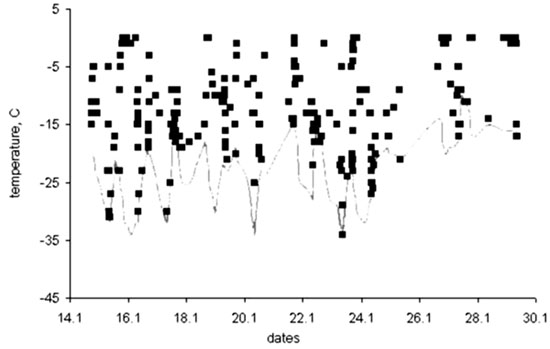
Fig. 4. Diurnal rhythm of the plumage surface temperature of wintering Sharp-winged Grouse; measured at the Lower Amur area, 52°N, 14–15 January 1997.
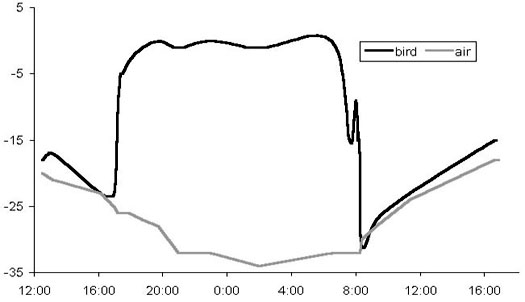
Fig. 5. Thermal blanket in the snow burrow of a Willow Grouse measured at the Kolyma upland area, 64°N, January 1986 (based on data from Andreev 1991a). Temperatures of the air and ground are indicated as are the specific heat flows (qb, qr, qs, qd in mw.cm–2)
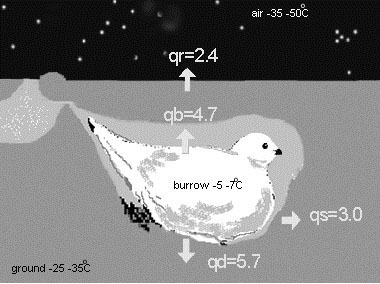
Fig. 6. Mass of woody droppings accumulated in the snow burrows of the Sharp-winged Grouse. Values as measured in the Lower Amur area, 15–28 January 1997.
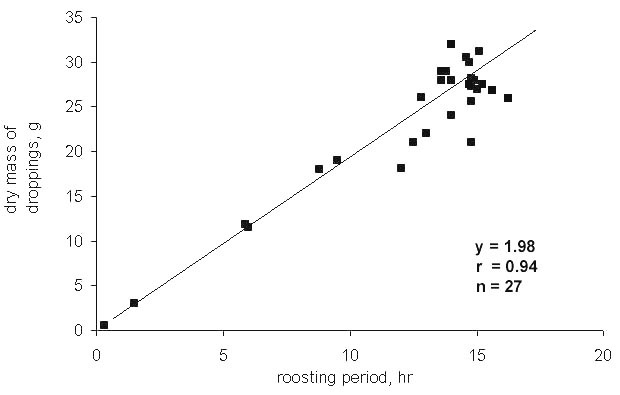
Fig. 7. Mass of accumulated woody droppings and reduction in crop weight in the snow burrows of Willow Grouse. Values for the Lower Kolyma area, 70°N, December 1980.
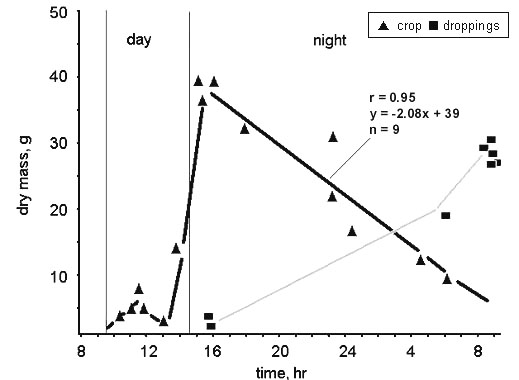
Fig. 8. Actual (triangles) and theoretical (line) metabolism in the Willow Grouse; values for December–March are from the Kolyma Upland, Lower Kolyma and Anadyr, for a bird of 600 g body mass (based on data from Andreev 1982).
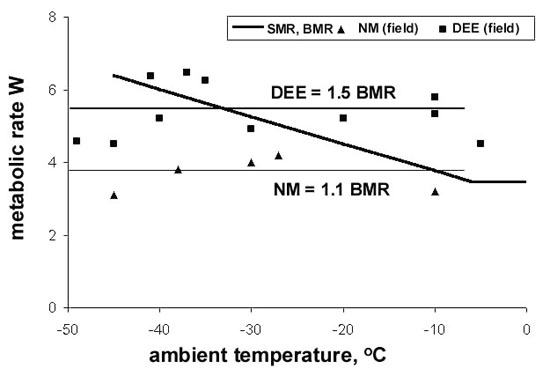
Fig. 9. Metabolic rates of Palearctic grouse in the wild: T.u. – Capercaillie, T.p. – Black-billed Capercaillie, F.f. – Sharp-winged Grouse, L.l. – Willow Grouse, L.m. – Rock Ptarmigan, T.b. – Hazel Grouse.
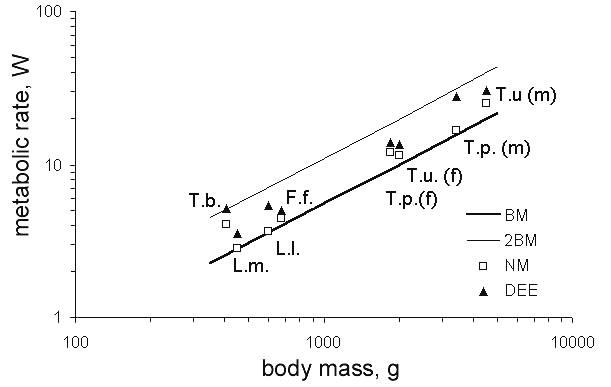
Fig. 10. Change in the body mass of the wintering male Willow Grouse; data for the Lower Kolyma area, September–May inclusive.
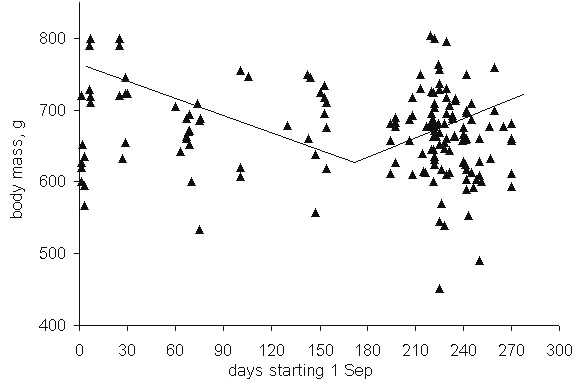
Fig. 11. Energy expended (DEE) and excreted (EE) daily by the two grouse species in mid- and late winter months. Figures in bar graphs give values for DEE in December and March. Calculations for the Sharp-winged Grouse refer to the Lower Amur region, for the Willow Grouse – to the Lower Kolyma.
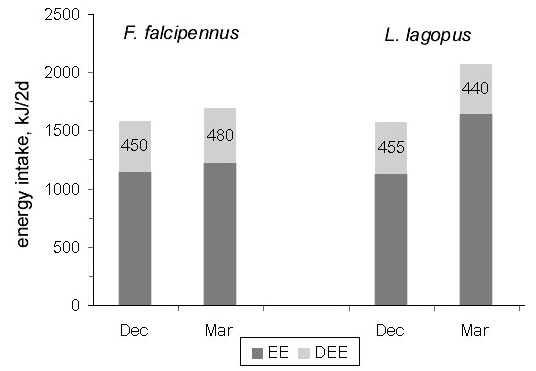
Fig. 12. Seasonal dynamics of the woody (Md) and caecal (Mc) droppings output in the Willow Grouse; sample size in brackets. Roman numerals are months of the year.
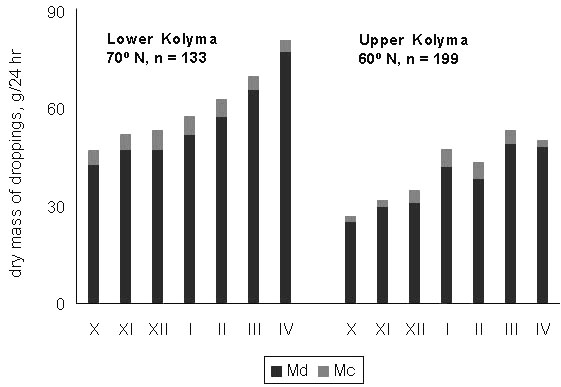
Fig. 13. Seasonal changes in the composition of winter diets of the Willow Grouse; data for the Lower Kolyma area.
Fig. 14. Chemical composition of the preferred and natural winter diets of the Willow Grouse; sample size in brackets.
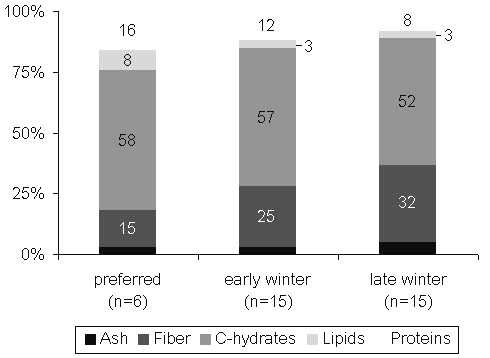
Fig. 15. Seasonal trend of the body mass in a wintering grouse (the average late summer weight referred as 100%).
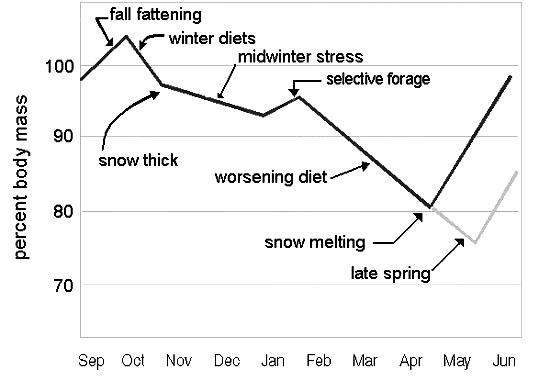
Fig. 16. Partitioning of energy expenditure of incubating birds: DEE – daily energy expenditure, DPEi – daily productive energy, DA – cost of activity, cdt – cost of thermoregulation, BM – basal metabolic rate, Qh – cost of rewarming clutch, Qi – cost of steady state incubation.
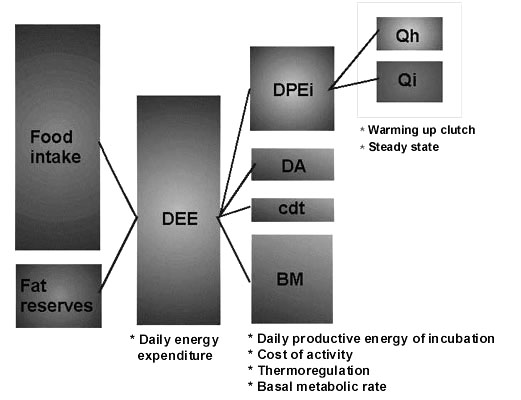
Fig. 17. Thermal parameters in the nest of the Sharp-winged Grouse during a 26h period, Lower Amur area, 23–24 May 1997.
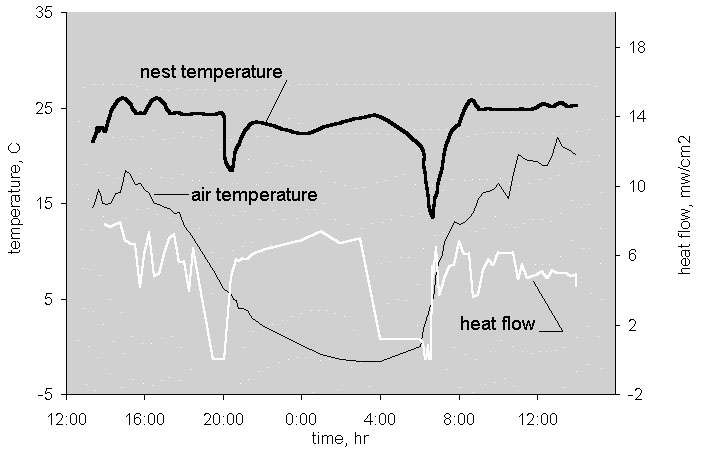
Fig. 18. Layout of sensors, typical temperatures, and typical patterns of heat flow of incubating waders in Arctic tundra, mid-June.
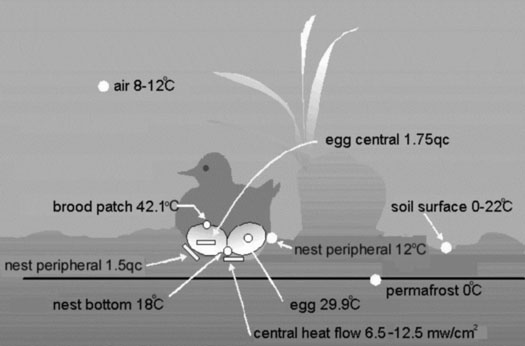
Fig. 19. Relationship between heat flow through clutch and the body mass of different Arctic wader species: C.t. – Temminck’s Sandpiper, Ph.l. – Red-necked Phalarope, Ph.f. – Red Phalarope, C.m. – Pectoral Sandpiper, Ch.m. – Mongolian Plover, Ph.p. – Ruff, P.d. – Lesser Golden Plover. Each triangle represents the average of at least 250 measurements under steady state incubation conditions.
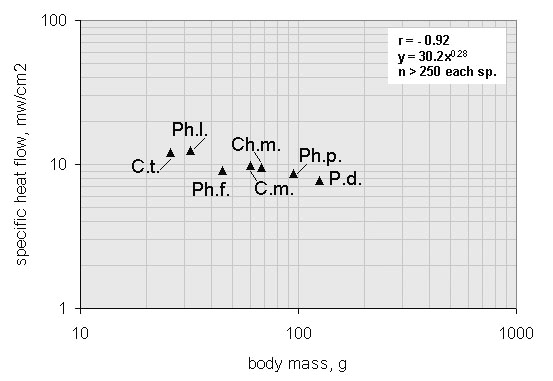
Fig. 20. Daily energy requirement of paired and single wader breeding species during mid-incubation period. Calculated for birds weighing 50 g. Abbreviations as on Fig. 16.
Fig. 21. Body mass of Pectoral Sandpiper females during the breeding season. Values are for birds captured on the Kolyma Lowland; gathered May through August 1979–1984.