Plenary03: Functional and evolutionary morphology of woodpeckers
Walter J. Bock
Department of Biological Sciences, Columbia University,1200 Amsterdam Avenue, Mail Box 5521, New York,NY 10027-7004, USA, fax 1 212 865 8246, e-mail wb4@columbia.edu
Bock, W.J. 1999. Functional and evolutionary morphology of woodpeckers. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban. Ostrich 70 (1): 23–31.
Woodpeckers are the first example of adaptive evolution by Natural Selection mentioned by Darwin who commented that their ‘ feet, tail, beak and tongue’ are ‘so admirably adapted to catch insects under the bark of trees’. Over the ensuing decades, the adaptiveness and evolution of these diverse woodpecker features has been examined by many workers but with limited success. A major problem was that these evolutionary explanations were not supported by logically prior functional explanations. Structures of the toes, hind limb and tail are associated with climbing. All forces acting on the climbing bird can be determined with the Method of Free-Body Analysis, which together with measurements of hind limb and tail features and of the tree surfaces used by different species, will permit understanding of the evolution of climbing adaptations. Bill shape and M. protractor pterygoidei development correlate with forces on the bill during drilling. Again Free-Body Analysis shows that compressive shocks acting on the bill do not travel directly into the brain case and hence the brain, but result in a compressive stress in the base of the skull. The frontal overhang in specialised drilling woodpeckers provides a bony stop that prevents excessive abduction of the upper jaw during non-impact periods while drilling into trees. Specialisations of the tongue are connected with greater protraction of the tongue to obtain food. These features not only include longer protractor and retractor muscles, but a universal joint between the basihyal and the fused paraglossalia and the enlargement of two pairs of intrinsic muscles inserting on the paraglossalia enabling movement of the tip of the corneous tongue in all directions. With a better understanding of the adaptiveness and evolution of these diverse features of woodpeckers, it is possible to obtain an improved comprehension of their ecological associations, adaptations and evolutionary history.
INTRODUCTION
In the Introduction to his On the Origin of Species, Darwin cited woodpeckers as the very first example to illustrate the evolutionary origin of adaptations, saying ‘. . . the woodpecker, with its feet, tail, beak and tongue, so admirably adapted to catch insects under the bark of trees’ and asks the question of how these excellent, but not perfect, adaptations came into being. Indeed woodpeckers not only possess a most outstanding suite of adaptive features, but the diverse species within this avian family display a broad spectrum of modifications of these adaptive features. In this paper, I would like to examine some of the adaptive features in woodpeckers for climbing, drilling into trees, and capturing food with their tongue. I will stress the functional analyses required for the assessment of the adaptiveness of these several features for climbing, pecking and food-catching, and will indicate how these functional-adaptational analyses serve as the foundation for further ecomorphological and evolutionary studies. Further, I will emphasise that functional explanations in biology are logically prior to and essential for evolutionary explanations, that is, in the absence of proper functional explanations, evolutionary explanations (including classifications and phylogenetic analyses) lack the necessary foundation and are vacuous. Most importantly, I would like to show that there are still many new developments and many interesting things to learn in the old field of avian morphology.
CLIMBING
Analysis of climbing in woodpeckers will include the arrangement of the toes, investigation of the mechanical forces on the climbing bird, and structure of the hindlimb muscles. I will not cover in detail the stiffened tail feathers in members of the Picinae which provide support when the bird is climbing (see Short 1982 for details). In most picines the two central tail feathers are stiffened, although some as Sapheopipo, Meiglyptes and Hemicircus, have only somewhat stiffened central tail feathers. Specialised forms, such as Campephilus, Chrysocolaptes, Reinwardtipicus and Blythipicus have the two central pairs of rectrices stiffened with a very concave (below) and strengthened vane. Jynx possesses a soft and non-supportive tail and the piculets have a very short tail that does not contact the tree similar to nuthatches.
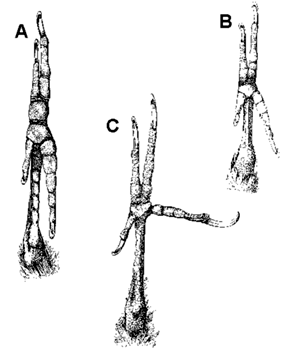
Woodpeckers, as all members of the Piciformes, possess a zygodactyl foot or ‘ yoke arrangement’ of the toes in which the fourth toe is reversed to the rear of the foot (Scharnke 1930, 1931; Stolpe 1932; Steinbacher 1935). Modifications evolved in the condyle of the fourth toe to permit an efficient reversal of the tendons to this toe (Steinbacher 1935). Because woodpeckers possess a zygodactyl foot and because they are mainly climbing birds, most ornithologists concluded that the zygodactyl foot is an adaptation for climbing. Not so, as argued in the early part of this century by Waldron de Witt Miller (see Bock & Miller 1959) who showed that the zygodactyl foot is an adaptation for perching and that the arrangement of the toes in climbing woodpeckers is not zygodactyl. Most members of the Piciformes, Psittaciformes and Cuculidae, all of which possess a zygodactyl foot, are perching birds; the basic requirement of a perching foot is having opposing toes to provide a grip on the branch. In climbing birds, including those that cling to vertical surfaces, as many toes as possible should oppose the downward pull of gravity. When woodpeckers perch, such as commonly done by the wryneck (Jynx), the toes are held in the zygodactyl arrangement. But when climbing, the fourth toe of woodpeckers is not reversed to the rear of the foot, but is extended laterally (Fig. 1 & Fig. 2). The lateral extension of the fourth toe (Fig. 1C) provides support against any sideward directed forces when the bird is climbing or drilling into the tree. Generally the short hallux (first toe) does not provide any support, and is reduced or lost in several genera of woodpeckers. In some genera, such as in the New World Ivory-billed Woodpeckers (Fig. 2), the hallux is long and is held to the lateral side or even positioned in the front of the foot when the bird is climbing.
The claws of the toes in woodpeckers must be strongly curved and sharply pointed so that they can penetrate into the bark when the bird is climbing trees and when the ventral surface of the toes is pressed against the bark surface. Strongly curved claws are also found in both the foot and the hand of Archaeopteryx and have been used as important evidence supporting the argument that this bird was arboreal and used both its forelimbs and hindlimbs to climb up trees (Feduccia 1996).
With woodpeckers climbing on the trunks and branches of trees, the next questions would be: What are the mechanical forces acting on a climbing woodpecker? And how does the placement of the feet and tail affect these forces? Forces on a climbing woodpecker were analysed by earlier workers (Stolpe 1932; Bock & Miller 1959), but erroneous assumptions were made on the direction and placement of various forces and incorrect physical model were used (Fig. 3). A proper model was presented by Winkler & Bock (1976; see also Bock & Winkler 1978) employing the method of free-body diagrams. This model includes both the rotational and linear displacement effects of all forces acting on a climbing bird, as well as the frictional forces at the contacts between the bird and the tree (Fig. 4). From this analysis, it is possible to show what measurements are needed to undertake a full biomechanical analysis and to calculate all forces acting on a climbing woodpecker. Moreover, one can inquire into the consequences of modifying various factors, such as holding the body closer or further from the tree, varying the length of the tail, or climbing without a supporting tail as in piculets. Free-body analysis shows the incorrectness of any model in which the position of the forces acting on the legs and the tail of climbing birds are assigned in an arbitrary manner (e.g. along the axis of the leg or tail or through the articulation of the leg with the limb girdle). Any method ignoring frictional forces at the contact points (i.e. contact forces placed at right angles to the contact surface) is also wrong.
The free-body model for the forces on a climbing woodpecker indicates that the flexor muscles of several segments of the hindlimb should be larger in cross-sectional area (i.e. develop more force) to hold (flex) the limb segments together against the effects of the gravitational force on the bird. One indication of this development is the absence of the M. accessorius semitendinosi in specialised drillers (Picoides, Sphyrapicus, etc.) although this muscle is present in less specialised taxa (Burt 1930: 502). Unfortunately nothing is known about the relative size and hence force development of the hindlimb muscles, especially the flexors, in the different species of woodpeckers with respect to their climbing specialisation.
Some years ago, Selander (1963) and Selander & Giller (1966) showed that different species of woodpeckers and even the two sexes of the same species used different parts of the tree for foraging, and that these diverse foraging patterns resulted in modifications in foot and tail structure. Climbing on trunks, branches and twigs of differing diameters represents a significant part of the niche requirements of the two sexes and of the individual species; one that can be readily analysed with the methods of ecomorphology (Leisler & Winkler 1985, 1991). Understanding the functional significance of toe arrangement during climbing and analysis of the forces on climbing woodpeckers permit a better appreciation of the measurements, including lengths of the tail, hindlimb, position of the bird on the tree, the hindlimb muscles, to be taken in such an ecomorphological study as well a better foundation for analysing the results.
DRILLING
If you bang your head against the wall, it feels good only when you stop – which leads to the commonly asked question of why don’t woodpeckers get headaches when they are drilling into trees? Or to put it in a more serious way, how do woodpeckers protect their brain from damage resulting from the impact of their bill tip when drilling into trees? A number of suggestions have been made on the possible existence of shock-absorbing mechanisms based on cranial kinesis and stretching of contracting muscles (Bock 1964: 29–30; Spring 1965). These shock-absorbing mechanisms work by increasing the duration of the impact and thereby reduce the maximum impact force. Such mechanisms, however, would reduce the impact force by the woodpecker on the tree and, hence, result in reduction in the efficiency of drilling. Suggestions of shock-absorbing mechanisms to reduce forces acting on the brain case and brain of woodpeckers during drilling can, therefore, be rejected. The consequences of the reaction forces by the tree on the bill during drilling can only be understood in terms of avian cranial kinesis (Bock 1964). For the sake of brevity, I will analyse only the forces acting on the upper jaw, as these relate most directly to possible damage to the brain, although the forces on the lower jaw can be readily added to the analysis.
Let us examine the structure of the woodpecker skull, and especially the kinetic hinge between the rostral end of the brain case and the caudo-dorsal base of the upper jaw (Fig. 5 & Fig. 6). Earlier workers analysing the forces on the woodpecker bill during drilling have overlooked this hinge and have shown a compressive shock passing directly from the upper jaw into the brain case. However, given that the upper jaw can rotate relative to the brain case around the nasal-frontal hinge, the actual consequences of the impact force are quite different. Let us consider the instance of impact of the woodpecker bill on the tree and all forces on the upper jaw using a free-body analysis (Fig. 7). For the purposes of this analysis, I will assume that the upper jaw is static with respect to the brain case at the moment of impact. The forces on the upper jaw will tend to rotate it and will tend to move it in some linear direction, hence equations are needed for both the rotational and the linear effects of the forces acting on the upper jaw. Forces on the upper jaw (Fig. 7) are: (a) the reaction (= impact) force (Fi) of the tree on the tip of the jaw, which will rotate the upper jaw in a clockwise direction around the nasal-frontal hinge; (b) the force (Fp) resulting from the contraction of the M. protractor pterygoidei and acting at the caudo-ventral base of the upper jaw which will tend to rotate the upper jaw in a counterclockwise direction; and, (c) the force (Fp) of the brain case acting on the upper jaw at the nasal-frontal hinge which does not rotate the upper jaw because this force passes through the center of rotation of the upper jaw. Because I have assumed that the upper jaw is static, the sum of the torques (moments) of Fi and of Fp must be equal to zero as shown in the equation for the sum of the moments (SMo). Inspection of the free-body diagram and the moment equation shows that moment arm ‘oa’ of force Fp is less than moment arm ‘ob’ of force Fi; hence, force Fp must be greater than force Fi. Again because the upper jaw is static, the sum of the linear affects of the forces, Fi, Fp, and Fa, must equal zero as shown in the equation for the sum of the linear effects of these three forces (SFx). Hence the vector direction of the force of the brain case on the upper jaw at the nasal-frontal hinge is directed caudad as shown; this is a tensile force of the brain case on the upper jaw (see Bock 1974). That is, the bone comprising the nasal-frontal hinge is under a tensile stress, not under compression as concluded by almost all earlier workers. The brain case is being pulled away from the upper jaw at the moment of impact of the bill tip with the tree, under the assumption that the upper jaw is in static equilibrium with respect to the brain case at the time of impact. Pulling apart of the brain case and the upper jaw ensues from the contraction of the M. protractor pterygoidei which pulls the brain case backward and pulls the pterygoid (and hence the rest of the bony palate and the upper jaw) forward because of the positions of its origin and insertion. The existence of a tensile stress at the nasal-frontal hinge means that a compression shock cannot exist in this position at the moment of impact of the bill tip with the tree. Co-parallel tension and compression cannot exist at the same location in a bone at the same time. And therefore no compression shock passes from the upper jaw into the brain case and to the brain. The consequence of the large impact force by the tree on the tip of the upper jaw is a compression between the origin and insertion of the M. protractor pterygoidei in the bone below the brain (Fig. 7). As we will see below, compression in the skull base is a continuous stress, not a suddenly appearing shock.
A comparison of woodpecker species reveals that with increasing specialisation for drilling, several changes occur in the structure of the bill. In less specialised forms, the bill is slightly decurved and is deep in the ventro-dorsal direction. With specialisation, the bill becomes straighter and ventro-dorsally compressed. These changes bring the vector lines of the impact force (Fi) and the protractor force (Fp) more in line which reduces the force at the nasal-frontal hinge (Fa) to a minimum. The bill also becomes wider at its base which provides a better support for laterally directed components of the impact force, but this is another aspect of the analysis that I shall not to cover here.
This functional significance of avian cranial kinesis, in which the resulting force in the upper jaw-brain case system is shifted from one part of the brain case to another, does not require any movement of the upper jaw, just the existence of the kinetic hinge and associated muscles. This should be always kept in mind because it is generally assumed that the functional properties and adaptive significance of avian cranial kinesis depend on movement of the upper jaw.
As shown above, the brain of woodpeckers is not affected by large compression shocks when the bird is drilling into a tree. What happens to the brain of woodpeckers when they are drilling into trees is still unknown. It can be assumed the brain case, as well as both eyes, decelerates rapidly when the bill hits the tree. If the brain is not firmly anchored to the inside of the brain case, it will move forward and hit the inner wall of the rostral part of the brain case at the moment of impact just as people are thrown forward when a car or train stops suddenly. Hitting of the brain against the front wall of the brain case could well result in damage to the brain, but we simply do not know the exact morphological relationships of the brain to the brain case, how tightly the brain fits into the brain case and whether the brain is held by a harness comprised of the dura mater, to be able to understand just how the woodpecker brain escapes injury when the bird is drilling into the tree.
Still another problem exists in this story, namely that of the contraction of the M. protractor pterygoidei. If this muscle contracted only after detection of the impact with the tree by sense organs and this information is transmitted to the brain followed by processing of this information in the brain and finally sending out motor nerve impulses to stimulate the M. protractor pterygoidei, then the impact would be long over before the necessary muscle force would develop. Hence, the M. protractor pterygoidei has to contract continuously when the bird is drilling into a tree, which leads immediately to the question of what happens to the upper jaw during the non-impact intervals? What prevents excessive upwards rotation (protraction) of the upper jaw during the non-impact intervals, thereby averting possible damage to the bone and other tissues of the nasal-frontal hinge? We must examine another structure found only in some woodpeckers, namely the frontal overhang of the brain case which lies directly over the thin flexible bone comprising the nasal-frontal hinge.
The frontal overhang was mentioned briefly by Shufeldt (1900: Fig. 2, p. 587), but he did not consider it further. This feature was discussed in detail by Burt (1930: 470–477, Fig. 2) who showed that this feature varied considerably among woodpeckers from non-existence to well developed, and that the development of the frontal overhang varies with the degree of specialisation of each species for obtaining food by drilling (Fig. 8). Burt did not provide a functional explanation, or a proper morphological description as he never showed the relationship between the frontal overhang and the nasal-frontal hinge. His discussion of the possible adaptational significance of the overhang is vague at best, comparing the woodpecker condition with ‘telescoping’ of the skull in whales (Burt 1930: 475). He did, however, demonstrate a correlation between the variation in the overhang and the environmental interactions of the several species of woodpeckers included in his study.
The frontal overhang is a bony stop that prevents the upper jaw from rotating too far upwards by the force of the M. protractor pterygoidei during non-impact intervals (Fig. 9). But this overhang is not the only feature that prevents excessive upward rotation of the woodpecker upper jaw. Combined action of the occipitomandibular ligament between the ventral edge of the occipital plate and the posterior surface of the medial process of the mandible and the M. pterygoideus would also prevent excessive upward rotation of the upper jaw during non-impact intervals, but this is an indirect system and requires expenditure of muscular energy. This ligament-muscle system is found in most or all birds, and may have worked originally in less specialised species of woodpeckers lacking the frontal overhang. The frontal overhang originated (possibly several independent times) and becoming increasingly developed in those species that have specialised for obtaining food by drilling. During a bout of drilling, the M. protractor pterygoidei contracts continuously and provides a constant protractor force on the upper jaw. During the non-impact intervals, the forces on the upper jaw are describe by the lower set of equations (Fig. 9). They change to the upper set of equations with the occurrence of the impact and the rise of the impact force, and then back again to the lower equations with the fading away of the impact force. Depending on the magnitude of all forces, one result of the different consequences of the forces during the impact and the non-impact intervals can be a slight depression (impact time) and elevation (non-impact interval) of the upper jaw which I have been able to observe in high-speed motion pictures.
An entire series of comparative ecomorphological analyses of different species, as well as sexual dimorphism (see Wallace 1974), are possible from this functional explanation, including the shape of the upper jaw from being somewhat decurved, dorso-ventrally deep, and laterally narrow with no frontal overhang in less specialised drilling woodpeckers to straight, dorso-ventrally shallow and laterally wide with a well-developed overhang in more specialised species. It is more difficult to say at this time what evolutionary changes may occur in a unspecialised drilling woodpecker that evolved from a specialised ancestor. That is whether there will be a reversal of the modifications in the structure of the upper jaw back to the conditions seen in primitively unspecialised forms.
CAPTURING FOOD
Most or all woodpeckers obtain their food with their tongue which can be protruded out of the mouth a short to a very long distance. The tip of the tongue may possess a series of barbs to spear food items or it may be coated with a sticky mucus from large salivary glands to glue these food items. I will not consider further the large and well-known mucus secreting salivary glands of woodpeckers. Not only can woodpeckers protrude and retract their tongues, but they have considerable control over the movement of its tip and hence the direction that the tongue will take in following the curves of an insect tunnel. I will consider each aspect of tongue function separately.
The muscles that protrude the tongue out of the mouth to capture food and retract it back into the mouth must be sufficiently long to move the tongue the required distance (Bock 1974, 1991). These muscles have been first described in full detail by Leiber (1907). For woodpeckers that protract their tongue a great distance, these muscles must be very long, at least three times as long as the distance that the tongue is protruded. In order to do so, these muscles must change their attachments to increase the distance between their origin and insertion. The muscles involved are the protracting M. branchiomandibularis (M b m; Fig. 10 & Fig. 11) and the retracting M. cricohyoideus (M cr h; = M. ceratotrachealis of Burt 1930: 511; Fig. 11). The usual retractor muscle of the tongue in birds (M. stylohyoideus; m st h; Fig. 11 & Fig. 15) is vestigial to absent in woodpeckers. Reduction in the M. stylohyoideus appears to have resulted because this muscle did not (or could not) increase its length by a change in its site of origin on the base of the skull, and thereby it lost its role as the major tongue retractor in the woodpeckers. Simultaneous elongation of the M. branchiomandibularis and the bones of the hyoid horn was essential for increased protraction of the tongue. The long hyoid horns and associated branchiomandibularis muscles curve around the skull and, in extreme cases, as in Picus (Fig. 10), enter the right nostril and extend to the rostral end of the space inside the upper jaw. In woodpeckers with an extremely long tongue, the hyoid horns and muscles may loop ventrally below the head before passing around the brain case (Fig. 10). The paired cricohyoideus muscles have also elongated and serve as retractors of the tongue. These muscles do not originate from the larynx as in most other birds, but from a more caudad point on the trachea. Increased length of these muscles is achieved by their winding around the trachea several times from their origin to where they pass forward to insert on the rostral end of the basihyal. The paired muscles may interweave with each other as they wrap around the trachea.
Most interesting is a ‘new’ skeletal muscle described by Leiber (1907), the M. esophagomandibularis (M e m; Fig. 11; = M. geniothyroideus of Leiber 1907: 39) which is an anterior slip of the muscular sheath of the oesophagus that extends to and inserts on the medial surface of the mandibular ramus. In unspecialised woodpeckers, this muscle still originates from the oesophagus, but in more specialised woodpeckers, its origin is from the larynx and/or the trachea; thereby, the M. esophagomandibularis has become a true skeletal muscle. The M. esophagomandibularis pulls the larynx and the cranial end of the trachea forward when the tongue is protruded from the mouth and thereby increases the reaching distance of the tongue.
The bony elements of the tongue apparatus change gradually from being thick and rigid at their rostral end to flattened dorso-ventrally and flexible along the hyoid horns (Fig. 12). When the muscles surrounding the hyoid horn, especially the M. branchiomandibularis contract, they apply force around the entire bone of the hyoid horn and change this bone from a flexible structure to a rigid one (Fig. 13 & Fig. 16). This is an example of a ‘hydrostatic’ structure, but with a bone filling rather than a fluid filling, which can transmit the force of the M. branchiomandibularis from its insertion on the distal tip of the hyoid horn along the entire length of this horn to the basihyal, and thereby push the corneus tongue out of the mouth.
Control of movement of the rostral tip of the corneous tongue depends on the structure of the articulation between the basihyal and the fused paraglossalia, and the insertions of the paired ceratoglossus (M c g) and the paired hypoglossus obliquus (M hg o) muscles. The articulation between the two bones is saddle-shaped (Fig. 14) so that this joint is a universal one, permitting the fused paraglossalia to move in any direction relative to the basihyal. The paired ceratoglossus muscles (M c g) insert at the two ventrolateral corners of the fused paraglossalia while the paired hypoglossus obliquus muscles (M hg o) insert at the two dorsolateral corners of this bone (Fig. 14 & Fig. 15). Both pairs of muscles are pinnate, possessing a large number of short fibers indicative of short excursion and large force applied to the bone at their insertions (Fig. 15). The M. hypoglossus obliquus usually originates only from the basihyal in birds, but in woodpeckers, it originates not only from the basihyal, but also from much of the ceratobranchiale (Fig. 13). With differential force application from each of these four muscles onto the four caudo-lateral corners of the fused paraglossalia, the tip of the corneous tongue can move in all possible directions relative to the basihyal and the rest of the tongue (Fig. 16). Hence the tip of the tongue can change its direction as the insect tunnel turns and, thereby, can direct movement of the tongue through the tunnel. Presumably touch organs exist in the tongue tip, which can detect the walls of the tunnel and transmit this information to the brain, and hence to differential stimulation signals in the motor nerves to these two pairs of muscles.
Diverse species of woodpeckers differ in the distance they probe with their tongue for their food, from very short in the sapsuckers (Sphyrapicus) to very long in the green woodpeckers (Picus) and the flickers (Colaptes). The lengths of the hyoid horns and of the branchiomandibularis and the cricohyoideus muscles increase as the woodpeckers evolve longer probing abilities and shorten secondarily if they evolve shorter probing foraging methods. Hence, it would be difficult to impossible to ascertain the primitive state of these muscles in the Picidae and especially in the Picinae. Modifications also exist in the degree of specialisation of the M. esophagomandibularis with this muscle inserting more directly onto the trachea in woodpeckers having increased specialisation for longer probing. Moreover, the vestigial M. stylohyoideus became smaller and finally disappeared in woodpeckers with increased specialisation for longer probing. In the case of these last two mentioned muscles, one would not expect a reverse in their structure in woodpeckers that have become secondarily short probers.
Specialisation for drilling and for longer probing in woodpeckers are not tied to one another so that it is possible to have species that are specialised drillers, but short probers, or unspecialised drillers and long probers, etc. The consequences of these two types of specialisation is that the associated morphological modifications may interact with one another in interesting ways, requiring that one analyse the morphology of the woodpecker head from an integrated, constructional viewpoint in addition to a functional viewpoint.
In most woodpeckers with long probing tongues, the elongated hyoid horns and muscles extend around the entire brain case and enter the right nostril to extend to the rostral end of the cavity within the upper jaw. In a few forms, such as the North American Hairy Woodpecker Picoides villosus, the hyoid horns do not enter the right nostril, but instead encircle the right eye (Fig. 17; see also Coues 1884; Shufeldt 1900; Leiber 1907). Any attempt to explain the arrangement of the hyoid horns in the Hairy Woodpecker or the difference between that seen in most long-probing species with the hyoid horns entering the right nostril and that seen in the Hairy Woodpecker with the hyoid horns encircling the right eye only using functional explanations for the tongue apparatus would be doomed to failure. The answer is an integrated, constructional explanation based on functional explanations of the upper jaw for drilling combined with those explanations of the tongue for probing. The Hairy Woodpecker is a specialised driller and, hence, has a dorso-ventrally flattened upper jaw which reduces the articular force at the nasal-frontal hinge as discussed earlier. Consequently there is simply no space within the cavity of the upper jaw to accommodate the hyoid horns and muscles. With a lengthening of the hyoid horns in the Hairy Woodpecker for longer probing, the only way that these long hyoid horns can be accommodated is to encircle the right eye.
SOME CONCLUSIONS
With an understanding of the functional and adaptational significances of the diverse features associated with climbing (hindlimb and tail) or with feeding (jaw and tongue) in woodpeckers, it is possible to inquire into the evolution of these systems within this group of birds. Because most features of the hindlimb, tail, jaw, and tongue systems can undergo independent evolution or can undergo ‘reverse’ evolution with changes in climbing and feeding habits of these birds, many of these features do not serve as useful clues in classificatory and phylogenetic analyses of this group. For example, the frontal overhang could well have originated several times in the history of this family. And the elongated branchiomandibularis and cricohyoideus muscles could have shortened during woodpecker woodpeckers such as in the sapsuckers. These conclusions considerably reduce confidence in earlier papers on the classification of woodpeckers and related groups (Goodge 1972; Simpson & Cracraft 1981; Swiercrzowski & Raikow 1981; see also Olson 1983) which exclude all functional analyses of the features used in their systematic studies. The analyses of Short (1982) and Winkler et al. (1995) are based on sets of features for which the functional and adaptational significances are reasonably well understood and provide a classification in which one can have greater confidence.
The functional-adaptational conclusions presented herein can provide the foundation needed for further ecomorphological analyses of this avian family, allowing a better basis on which to chose the features to be measured and on which to interpret the statistical results.
What is still lacking is comparative information on the morphology of many genera of woodpeckers (Picinae), especially of tropical genera, of the wrynecks (Jynginae), and of the piculets (Picumninae); almost nothing is known about the last two groups. Although most ornithologists assume that the wrynecks and the piculets are primitive groups within the Picidae, there is little to no evidence supporting this conclusion. The piculets could be an advanced group within the Picidae which has evolved a climbing habit excluding the use of the tail as a brace. And the wrynecks could be another advanced group specialised on feeding on non-tree burrowing insects for which climbing on vertical surfaces was no longer required; therefore, the use of the tail as a stiff prop during climbing decreased during the evolution of this subfamily. We simply do not have any reliable evidence at present on which to reach a decision on whether these two subfamilies are primitive or advanced in the evolution of the Picidae.
My major conclusion is that there are still many new and interesting studies to do within avian anatomy. This does not mean only numerous fascinating and hitherto unknown features to describe, of which there are still many. Good examples are the two different types of secondary articulations of the lower jaw in many birds and the secondary occipital condyle characteristic of the hornbills. Of greater importance are the development and application of new methods of functional and adaptational analyses, such as the method of free-body diagrams which can be applied to almost every type of biomechanical analysis of the skeletomuscular system. All of these studies depend on carefully done morphological descriptions, but they also depend on looking outside of traditional morphological studies for useful methods of functional analyses, on being a biological engineer to think about how these structures can work, on being a naturalist to think about how these functioning morphological systems are used in the life of birds, and on being a comparative biologist and evolutionist to think about the medley of evolutionary explanations that can be formulated on prior descriptive and functional analyses. With all that has been learned about the physiology, behaviour and ecology of birds over the past century, it is possible to return to the study of avian structure and develop a real biological and evolutionary avian morphology. Without question, the golden age of avian anatomy is still ahead of us.
ACKNOWLEDGEMENTS
I would like to express my appreciation to the Scientific Program Committee for their invitation to present a Plenary Lecture at the 22nd International Ornithological Congress. And I wish to thank the two referees, Professor Dominique G. Homberger and Professor Hans Winkler, for their careful review of the manuscript and for their excellent suggestions for improving the manuscript.
REFERENCES
Bock, W.J. 1964. Kinetics of the avian skull. Journ. Morph. 114: 1–42.
Bock, W.J. 1974. The avian skeletomuscular system. In: Farner, D.S. & King, J.R. (eds) Avian biology. Vol. iv, Chap. 3: 119–257.
Bock, W.J. 1991. Levels of complexity and organismal organization. In: Lanzavecchia, G. & Valvassori, R. (eds) Form and Function. Selected Symposia and Monographs U.Z.I., 5: 181–212; Italy: Mucchi, Modena.
Bock, W.J. & de W. Miller, W. 1959. The scansorial foot of the woodpeckers, with comments on the evolution of perching and climbing feet in birds. Amer. Mus. Novitates, 1931, 45 pp.
Bock, W.J. & Winkler, H. 1978. Mechanical analysis of the external forces on climbing mammals. Zoomorphologie 91: 49–61.
Burt, W.H. 1930, Adaptive modifications in the woodpeckers. Univ. Calif. Publ. Zool., 32(8): 455–524.
Coues, E. 1884. Key to North American Birds. Second Edition. Boston, MA: Estes and Lauriat.
Feduccia, A. 1996. The Origin and Evolution of Birds. New Haven, CT: Yale Univ. Press.
Leiber, A. 1907. Vergleichende Anatomie der Spechtzunge. Zoologica (Stuttgart), No. 51.
Leisler, B. & Winkler, H. 1985. Ecomorphology. In: Johnston, R.F. (ed.) Current Ornithology, Vol. 2: 155–186. New York and London: Plenum Press
Leisler, B. & Winkler, H. 1991. Ergebnisse und Konzepte ökomorphologischer Untersuchungen an Vögeln. Journ. f. Ornith., 132: 373–425.
Olson, S. 1983. Evidence for a polyphyletic origin of the Piciformes. Auk 100: 126–133.
Scharnke, H. 1930. Physiologisch-anatomische Studien am Fuss der Spechte. Journ f. Ornith. 78: 308–327.
Scharnke, H. 1931. Über die Halluxrudimente bei dreizehigen Spechten. Ornith. Monatsber. 39: 33–37.
Selander R.K. 1963. Species limits in the woodpecker genus Centurus (Aves). Bull. Amer. Mus. Nat. Hist., 124: 213–274.
Selander, R.K. & Giller, D.R. 1966. Sexual dimorphism and differential niche utilization in birds. Condor 68: 113–151.
Short, L.L. 1882. Woodpeckers of the World. Greenville, MD: Delaware Museum of Natural History.
Shufeldt, R.W. 1900. On the osteology of the woodpeckers. Proc. Amer. Phil. Soc., Phila., 39: 578–622.
Simpson S.F. & Cracraft, J. 1981. The phylogenetic relationships of the Piciformes (class Aves). Auk 98: 481–494.
Spring, L.W. 1965. Climbing and pecking adaptations in some North American woodpeckers. Condor 67: 457–488.
Steinbacher, G. 1935. Funktionell-anatomische Untersuchungen über Vogelfüssen mit Wendezehen und Rhchzehen. Journ f. Ornith. 83: 214–282.
Stolpe, M. 1932. Physiologisch-anatomische untersuchungen über die hintere Extremität der Vögel. Journ f. Ornith. 80: 161–247.
Swiercrzowski E.V. & Raikow, R.J. 1981. Hindlimb morphology, and phylogeny and, classification of the Piciformes. Auk 98: 466–480.
Wallace, R.A. 1974. Ecological and social implications of sexual dimorphism in five melanerpine woodpeckers. Condor 76: 238–248.
Winkler, H & Bock, W.J. 1976. Analysis der Kräfteverhältnisse bei Klettervögeln. Journ. f. Ornith. 117: 397–418.
Winkler, H., Christie, D.A. & Nurney, D. 1995. Woodpeckers: a guide to the woodpeckers, piculets and wrynecks of the world. Sussex, England: Pica Press.
Fig. 1. Plantar surface of the foot to show the arrangement of the toes. (A) Pteroglossus (Ramphastidae; perching foot). (B) Celeus elegans (Picidae; perching and climbing foot). (C) Dryocopus pileatus (Picidae; climbing foot). From Bock & Miller 1959: Fig. 1

Fig. 2. Plantar surface of the foot to show moderate and extreme forward rotation of the fourth toe and hallux in the Ivory-billed Woodpeckers. (A) Campephilus melanoleucos. (B) Campephilus rubicollis. From Bock & Miller 1959: Fig. 6.
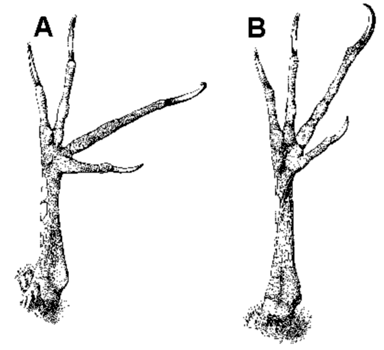
Fig. 3. Earlier model of forces acting on a climbing woodpecker redrawn from Stolpe (1932: Fig 37, p. 212) using his symbols but with conversions to the model of Winkler and Bock. The slight deviation of force vector ‘b’ from the longitudinal axis of the tail is copied carefully from Stolpe. From Winkler & Bock 1976: Fig. 1.
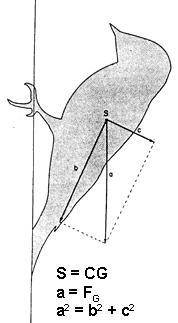
Fig. 4. The woodpecker (tailed) model: general case with the bird at rest clinging to an oblique (overhanging) surface. The tail contacts the tree at point ‘o’ and both feet at point ‘c’. The force of gravity (FG) acts at point ‘e’, the center of mass (CG). To be determined are the two unknown forces (heavy dashed arrows) between the tree surface and the tail (Ft) and the feet (Ff) with the minimum number of measurements. The force of gravity is set at 50 gm-wts, and F1 is arbitrarily assumed to be 30 gm-wts, hence F2 is 20 gm-wts. The results of the analysis are shown on the figure and summarised in the closed polygon of forces (# 1) of heavy arrows. Results of three additional analyses are summarised in the closed polygons of lighter dashed lines; for analysis 2, F1 = 20 gm-wts, for 3, F1 = 10 gm-wts, and for 4, F1= 5 gm-wts. From Winkler & Bock 1976: Fig. 2.
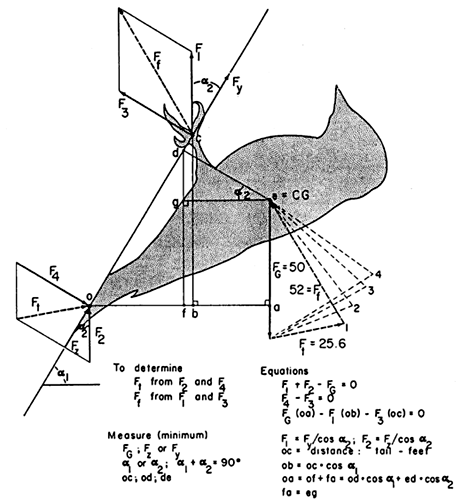
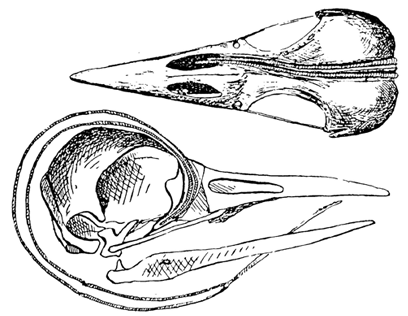
Fig. 5. Skull and hyoid apparatus of the Red-bellied Woodpecker Melanerpes carolinus in lateral view. (A) Braincase and upper jaw with the hyoid apparatus in place. (B) Mandible. (C) Pterygoid bone showing the large spine for the insertion of the M. protractor pterygoidei.
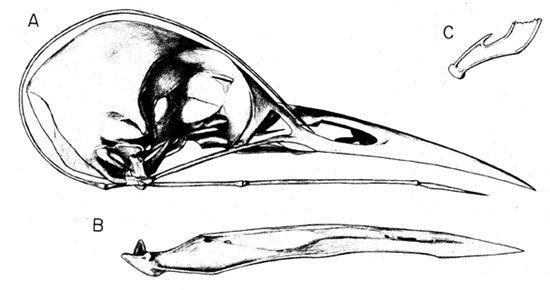
Fig. 6. Lateral view of the skull and hyoid apparatus of the Red-bellied Woodpecker Melanerpes carolinus showing the protractor muscles of the tongue and of the palate (pterygoid). Contraction of the tongue protractor (arrow A) results in protrusion of the tongue out of the mouth. Contraction of the palate protractor (arrow B) results in protraction (= elevation) of the upper jaw (arrow C).
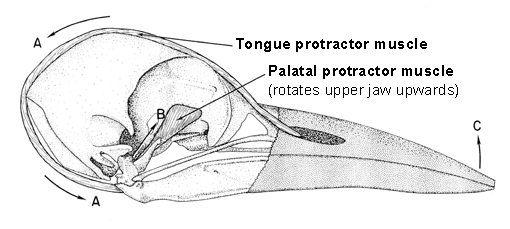
Fig. 7. Free-body diagram and associated equations showing the analysis of the forces on the upper jaw at the moment of impact of the jaw tip against a tree. The upper jaw is assumed to be static at the impact. Note that force Fa of the brain case on the upper jaw at the nasal-frontal hinge and its equal and opposite force of the upper jaw on the brain case (not shown) results in the bone of the nasal-frontal hinge being under tension. The consequence of the large impact force of the tree on the tip of the upper jaw (Fi) is a large compression force C in the base of the brain case below the brain between origin and insertion of the M. protractor pterygoidei.
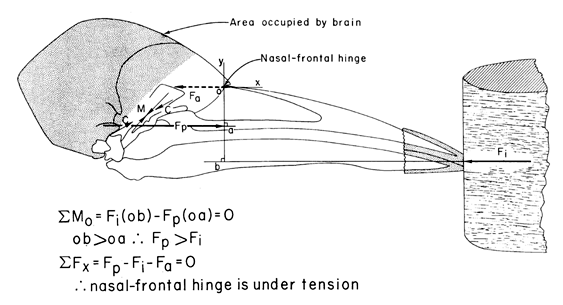
Fig. 8. Series of skulls of woodpeckers in lateral view (a – i) and upper jaw in dorsal view (a’ – i’) showing the change in the frontal overhang. The taxa shown are: (a) Picoides tridactylus or arcticus; (b) Picoides villosus; (c) Sphyrapicus varius; (d) Dryocopus pileatus; (e) Melanerpes carolinus; (f) Melanerpes formicivorus; (g) Melanerpes erythrocephalus; (h) Melanerpes lewis; (i) Colaptes auratus. These are arranged in order of the most specialised (a) to the least specialised (i) forms in obtaining their food by pecking into trees. Modified from Burt 1930: Fig. 2.
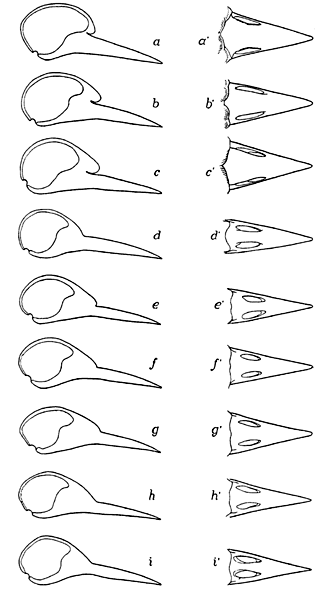
Fig. 9. Free-body diagram and associated equations showing the analysis of forces on the upper jaw at the moment of impact and during the non-impact periods. The frontal overhang as a bony stop and its relationship to the nasal-frontal hinge are shown in the insert; this bony stop prevents excessive elevation of the upper jaw. The equations for the impact time are as discussed in Fig. 10. A stout L. occipitomandibular connects the base of the brain case to the posterior end of the mandibular ramus. Two muscles, the M. pterygoideus and the M. pseudotemporalis profundus hold the upper and lower jaws together so that they act as a single unit. During the non-impact intervals, the moments of the resisting forces of the L. occipitomandibular (Fo) and/or the bony stop (Fs) prevent balance the moment of the palatal protractor force (Fp), and thereby prevent excessive dorsal rotation of the upper jaw.
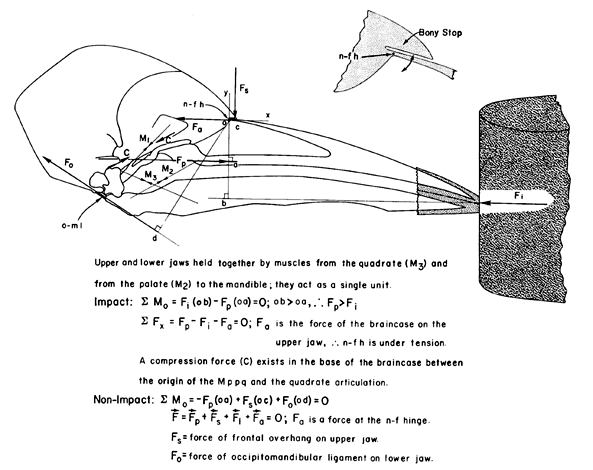
Fig. 10. Head of Picus viridis in lateral view to show the elongated hyoid horns and M. branchiomandibularis. Note the passage of both hyoid horns into the right nostril and cavity of the upper jaw. Also note the large mucus secreting salivary gland below and behind the mandible and brain case. From Leiber 1907: Fig. 10.
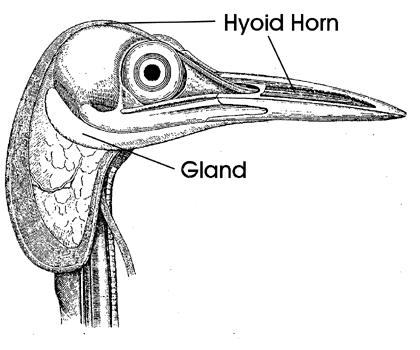
Fig. 11. Tongue muscles of Melanerpes carolinus in ventral view (A) and in dorsal view (B). Note the M. branchiomandibularis (M b m; tongue protractor), the M. cricohyoideus (M cr h; tongue retractor), the vestigial M. stylohyoideus (M st h), and the M. esophagomandibularis (M e m).
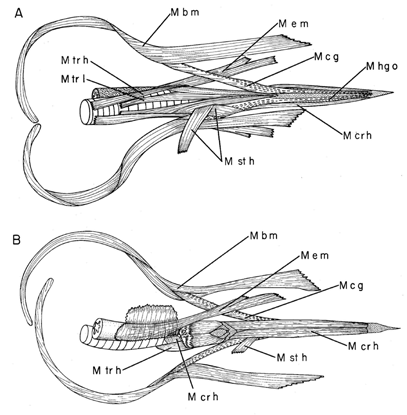
Fig. 12. Hyoid horns of Melanerpes carolinus to show the change in cross section from a thick and rigid anterior end to an increasingly thinner and flexible posterior end.
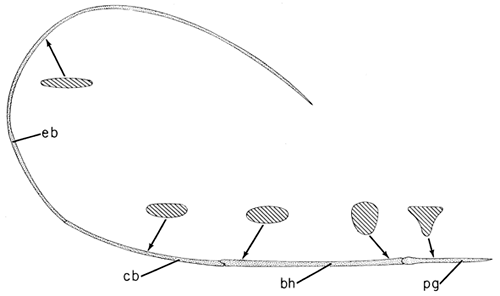
Fig. 13. Hyoid horn of Melanerpes carolinus to show the muscles completely encasing the thin bones of the hyoid. Note the M. branchiomandibularis (M b m; tongue protractor), the M. hypoglossus obliquus (M hg o), and the M. ceratglossus (M c g ) which are the two paired muscles rotating the tongue tip in all directions.
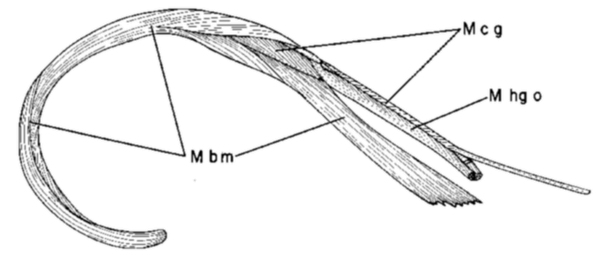
Fig. 14. Fused paraglossalia bones and the basihyal of Melanerpes carolinus in dorsal (A), lateral (B), and ventral (C) views to show the saddle-shaped articulation between these bones which permits movement of the fused paraglossalia in all directions relative to the basihyal. Note the insertion of the paired hypoglossus obliquus muscles (M hg o) at the two dorso-lateral corners and of the paired ceratoglossus muscles (M c g) at the two ventro-lateral corners of the fused paraglossalia.
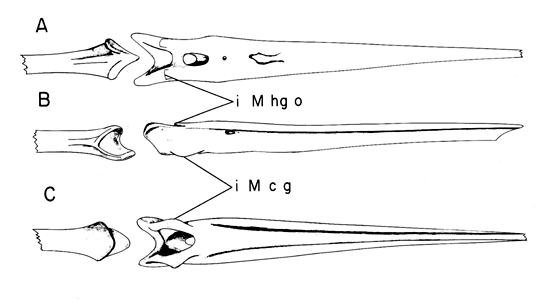
Fig. 15. Tongue muscles of Melanerpes carolinus in ventral view (A) and in dorsal view (B). Note the M. cricohyoideus (M cr h; tongue retractor), the M. hypoglossus obliquus (M hg o) and the M. ceratoglossus (M c g) which are the two paired muscles rotating the tongue tip in all directions. Also note the vestigial M. stylohyoideus (M st h; the usual tongue retractor in birds, but not in woodpeckers).
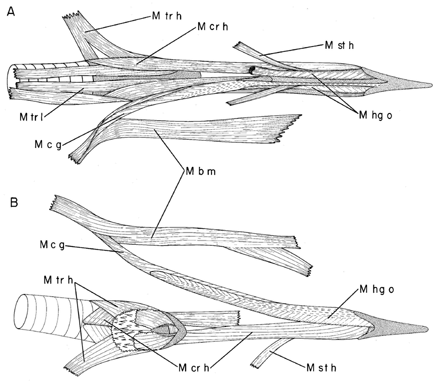
Fig. 16. Schematic model showing the rotation of the anterior tip of the woodpecker tongue by differential contraction and hence force development of the paired hypoglossus obliquus (M hg o) and paired ceratoglossus muscles (M c g). Protraction and retraction of the tongue by the branchiomandibularis (M b m) and cricohyoideus (M cr h) muscles is also shown as well as stiffening of the flexible bones of the hyoid horns by the squeezing forces of the surrounding muscles (M hg o and M c g; and more distally, the M b m, not shown).
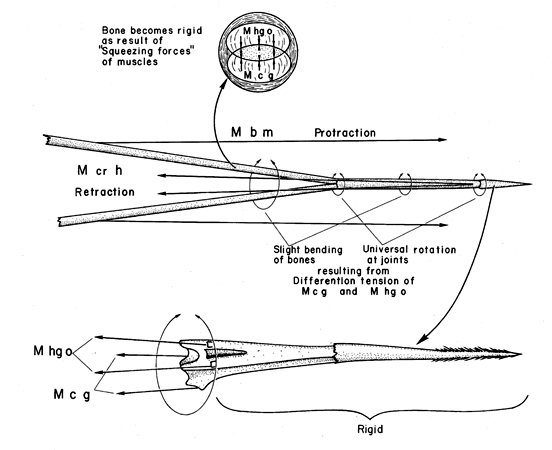
Fig. 17. Skulls and hyoid horns of Colaptes auratus (upper) and of Picoides villosus (not Picus as claimed by Coues and followed by Shufeldt) to show the different ways in which the elongated hyoid horns are accommodated in the head of woodpeckers. In Colaptes, these horns enter the right nostril and extend into the cavity of the upper jaw. In Picoides villosus, they curve around the right orbit. From Shufeldt 1900: Figs 6 and 7; originally from Coues 1884: Figs 73 and 74.