Plenary05: Experiments on competition between Great and Blue Tit: Effects on Blue Tit reproductive success and population processes
André A. Dhondt1& Frank Adriaensen2
1Laboratory of Ornithology, Cornell University, Ithaca, NY 14850, USA, fax 1 607 254 2415, e-mail aad4@cornell.edu; 2Department of Biology, UIA, University of Antwerp, B-2610 Wilrijk, Belgium, e-mail fadria@ua.uia.ac.be
Dhondt, A.A. & Adriaensen, F. 1999. Experiments on competition between Great and Blue Tit: Effects on Blue Tit reproductive success and population processes. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban. Ostrich 70 (1): 39–48.
Great Tits Parus major and Blue Tits Parus caeruleus compete for food and for cavities. By providing nest boxes with entrance holes small enough to exclude Great Tits, but allowing access to Blue Tits, either by themselves or in combination with standard nest boxes we manipulated the breeding densities of Blue and Great Tits independently. We compared populations differing in experimental densities over five-year periods. Components of Blue Tit reproductive success are adversely affected when either Blue Tit or Great Tit densities are experimentally increased. At high Blue Tit density adult survival does not change. When small-holed nest boxes are provided and Blue Tit density is increased, the number of local born males that recruit into the population increases considerably, so that the proportion of local born males is much higher than at low density. Similarly the local recruitment rate (the number of local recruits per adult breeding in the previous year) increases strongly for males, but not for females. The presence of small-holed nest boxes increases habitat quality, especially in winter when Blue Tits use these boxes for roosting, so that juveniles disperse less, and breeding density increases
INTRODUCTION
Keddy (1989, p. 2) defines competition as ‘the negative effects which one organism has upon another by consuming, or controlling access to, a resource that is limited in availability’. This definition is brief and precise and: (1) It reminds us of the fact that for competition to take place a resource must be limited in availability, i.e. there is not enough of it, or it takes too long to find it, or access to the resource is hindered by interactions with other individuals. (2) It tells us that the effect of competition primarily operates at the level of the individual.
The negative effects of competition operate at different levels of organisation. At the level of the individual competition can affect: (1) reproduction (delayed start of reproduction and/or reduced reproductive output in a single attempt, both resulting in a lower lifetime reproductive success); (2) survival (reduced chance to survive); (3) niche use or habitat selection (reduced access to high quality sites or habitat, resulting in a reduced reproductive success or a reduced chance to survive); (4) dispersal (increased likelihood that an individual will leave a site).
Individuals with different traits differ in ‘competitive ability’, and hence will be differently affected by competition.
The effects on individuals are translated into effects at the population level. When competition is more severe one or more population processes are adversely affected (reduced reproductive rate, reduced survival rate, decreased immigration rate, increased emigration rate). These effects on population processes further translate into lower density and/or relatively more young age classes (Hairston 1980), and result in a reduced per capita growth rate at a given density.
Populations subjected to different competition pressures should evolve to become different. Hairston’s (1980) very elegant field experiments using plethodonthid salamanders show that such may be the case. Differences between individuals can be in life-history traits, or in morphological/physiological/biochemical traits (Schoener 1983). In laboratory populations such evolutionary changes can be rapid. (Pimentel et al. 1965)
Finally, competition also has effects at the community level: Competition is assumed to influence community composition (number of species, and their relative abundance), and realised niche width (Wiens 1989).
Competition occurs over a limited resource. When the resource is less abundant or the number of competitors higher, the effect of competition should be more apparent and/or easier to detect. There are therefore two possible ways to study competition experimentally: (1) by manipulating the abundance of competitors and (2) by manipulating the limiting resource.
It tends to be relatively easy to reduce numbers, but more difficult to increase their abundance. In order to manipulate resource abundance one must assess which resource is in short supply, and be able to manipulate its abundance.
John Wiens (1989) reviewed the literature on field experiments in birds testing for the existence of interspecific competition. He concluded that manipulations of resources (6/12 providing evidence for the existence of interspecific competition) were apparently less successful to demonstrate competition than density alterations (16/23). It should be pointed out that in 5/12 experiments involving resource manipulations, food was added for some Parus species; and 11/23 experiments reporting density manipulations also involved Parids. An additional four studies used other hole-nesting species. Of the 35 studies reported by Wiens, 20 involved some cavity nesting species. In more recent studies cavity nesters have also played an important role. This suggests that parids are a good model to study competition experimentally because there is evidence for competition for food and for cavities, and because experimental manipulations are relatively easy.
A detailed review of the literature on field experiments concerning interspecific competition yielded some surprises.
(1) Very few studies exist in which population parameters have been compared in detail between populations subjected to different treatments over extended periods of time. Most studies only look at a limited number of population parameters, and most last a year or less.
(2) Most experimental manipulations involve a comparison between plots in which the numbers of an alleged competitor were reduced (experiment), or not changed (control). Very few, if any, experiments kept the numbers of one competitor constant, but increased the density of the other species by manipulating resources.
(3) Only 8/35 studies reviewed by Wiens (1989) were both replicated and had adequate controls.
(4) Nobody had asked whether or to what extent a change in interspecific competition in a field experiment could or would result in a rapid evolutionary response. In fact, Connell (1980, 1983) questioned whether any such co-evolutionary change between competitors would occur at all, because, unlike in interactions between trophic levels (i.e. predator–prey, parasite–host), the interaction between competitors is not continuous.
In west-European managed forests natural cavities are in short supply. When artificial nest boxes of appropriate dimensions are provided, the breeding densities of secondary cavity nesters, such as Great and Blue Tit can increase two- or threefold over the situation without these nest boxes (East & Perrins 1981; pers. obs.). The effects on Pied Flycatchers Ficedula hypoleuca can be even more dramatic (Lundberg & Alatalo 1992). Furthermore, adding nest boxes can also have indirect effects. Thus Red-cockaded Woodpeckers Picoides borealis were more successful when clusters of nest boxes were added, because they were less frequently excluded from their own cavities by Eastern Bluebirds Sialia sialis and Southern Flying Squirrels Glaucomis volans (Loeb & Hooper 1997). Adding nest boxes, thereby increasing the density of cavity nesters, can have negative effects on the abundance of open nesters through presumed interspecific competition (Hogstad 1975; Bock et al. 1992) or positive effects through presumed heterospecific attraction (Monkkonen et al. 1990).
COMPETITION BETWEEN GREAT AND BLUE TITS
When nest boxes suitable for both Great and Blue Tits were provided, Minot & Perrins (1986) found that the relative abundance of Blue Tits increased with the total density of the nest boxes until a certain threshold was reached. When nest boxes were superabundant Great Tit breeding density was limited by intra-specific competition, mainly for territories, and Blue Tit breeding density by interspecific competition, whereby there remained open space between Blue Tit territories (Dhondt et al. 1982). Blue Tits occupied the better quality areas only, better quality being defined as areas in which the birds laid a larger clutch (Dhondt et al. 1992)
Because Great Tits are larger than Blue Tits, one can regulate access to the next boxes by varying the size of the entrance hole. Large-holed boxes can be used by both species; small-holed nest boxes can be used by Blue Tits only. When given the choice in an aviary, Blue Tits preferred large-holed over small-holed nest boxes for roosting (Dhondt & Eyckerman 1980; Kempenaers & Dhondt 1991). When a Great Tit was added to the aviary, Blue Tits more frequently roosted in small-holed nest boxes (Kempenaers & Dhondt 1991). In forests, the number of Blue Tits found roosting in nest boxes during winter was very low when only large-holed boxes were present, and increased significantly when small-holed boxes were offered either by themselves, or together with large-holed nest boxes (Dhondt et al. 1991). In study plots with small-holed nest boxes (either by themselves or in combination with large-holed nest boxes) the breeding density of Blue Tits was 41–68% higher compared to plots in which only large-holed boxes were present (Dhondt et al. 1991). The fact that by manipulating nest box type during winter one can influence the subsequent breeding density of tits makes it possible to study the influences of both intra- and interspecific competition experimentally.
In all experiments we used high densities of wooden nest boxes with an entrance hole diameter of 32 mm (large-holed) and/or with an entrance hole diameter of 26 mm (small-holed).
Many studies have shown either through correlative analyses of long-term descriptive data sets, or through field experiments, that both intra- and interspecific competition during the breeding season can have an effect on reproductive traits of tits. Competition during the breeding season is mainly for food, since the diets of the two species overlap to a large extent. The intensity of competition, however, varied between studies. Many studies showed an effect of Blue Tit abundance on Great Tit reproduction, but the reverse was rarely studied (Dhondt 1989). We will here limit our discussion to intra- and interspecific effects on Blue Tits and refer to an earlier review for a summary of opposite effects (Dhondt 1989).
Very few studies have reported density-dependent effects on Blue Tit reproduction. Perrins (1990) found an inverse correlation between Blue Tit clutch-size and Blue Tit breeding density in two plots within Wytham Woods near Oxford, but not in a third. Dhondt et al. (1992) found such an effect in one of two plots. Van Balen & Potting (1990) found no density dependent effects on any trait in three study plots. Effects of Great Tit density on Blue Tit reproduction are even rarer. The only study reporting such an effect is that of Perrins (1990) who found an inverse relationship between Blue Tit clutch-size and Great Tit density in one of three plots near Oxford.
GOALS OF THE STUDY
Taking advantage of the fact that it is possible to manipulate the density of Great and Blue Tits independently, we wanted to test experimentally effects of interspecific competition between Great and Blue Tits, and possible effects of intra-specific competition among Blue Tits in a comprehensive and long-term study. As concerns competition during the breeding season we replicated experiments in habitats of different quality, to determine to what extent competition would be more intense in poorer quality breeding conditions, where resources should be more limiting. Since we can ‘create’ Blue Tit populations at higher and lower densities, we also wanted to determine which of the four population processes would be mainly responsible for generating and maintaining the high densities.
METHODS
Study plots
The experiments were carried out in two sets of study plots in Northern Belgium about 60 km apart. More details on these plots can be found in Dhondt & Eyckerman (1980), Dhondt et al. (1984) and in Dhondt (1989b). The names in brackets are those used in the above references, if different from those used in this paper.
Ghent: all plots are isolated fragments.
(1) Plot MA (Maaltepark): 10 ha; mixed deciduous suburban park, with a few conifers. 79 large-holed nest boxes between 1963–1978; an additional 50 small-holed nest boxes 1979–1983.
(2) Plot SO (Soenen): 8 ha; mixed deciduous rural park. 89 large-holed nest boxes 1967–1977 and 1979–1983. 80 small-holed and 9 large-holed nest boxes in 1978.
(3) Plot GT (Gontrode): 18 ha; mixed deciduous, mainly oak; rural. 108 large-holed nest boxes 1972–1976; 98 small-holed and 10 small-holed 1977–1983.
(4) Plot HP (Hutsepot): 27 ha; mixed deciduous, mainly beech; rural. 184 large-holed nest boxes 1964–1983.
Antwerp: plots are within a large forested area:
(1) Plot T: 12.5 ha; mixed deciduous, mainly oak, in the ‘Peerdsbos’, a large wooded estate of ca. 150 ha; 80 (1979), then 120 (1980–1983) large-holed nest boxes; 120 small-holed nest boxes (1984–1988); 120 large-holed and 60 small-holed nest boxes 1989–1993.
(2) Plot B: 12.5 ha mixed deciduous, mainly oak, in the ‘Peerdsbos’ at 600 m from Plot T; 59 small-holed and 118 large-holed nest boxes 1979–1993 (in 1979 only 59 large-holed boxes).
(3) Plot L: 7.5 ha; mixed deciduous park, adjacent to Plot T. 50 large-holed nest boxes 1980–1988.
During the breeding season boxes were checked once a week and their contents noted, providing information on laying-date, clutch-size, number of hatched young, and number of fledglings. All fledglings were banded, and in Antwerp the young were also weighed and their tarsus measured at 15 days of age. Adults were trapped when feeding young and identified or banded, weighed and measured.
During winter the nest boxes were checked at least twice, and birds identified or banded.
All measurements of the same individual bird were compared, and if necessary, correction factors for systematic observer differences calculated and applied. Breeding season weights are used without corrections; tarsus length and wing length are used after correcting for observer.
Manipulation of Blue Tit densities in this study – description of the basic experimental approach
The basic experimental approach used was as follows: We chose pairs of study plots and compared these during two periods of 5 years each. In the control plot the experimental setup did not change between the periods. In the experimental plot the nest box configuration was changed between the two periods, resulting in a different breeding density of one species. Table 1 summarises the method used to describe nest box configurations in this paper. A significant plot*period interaction term, in a PROC MIXED SAS procedure (SAS Institute 1997), with year randomised within period, was considered evidence of competition.
We compared the following reproductive traits:
(1) Julian laying date: Julian date when the first egg was laid in complete first clutches.
(2) Clutch-size: Mean number of eggs laid in complete first broods.
(3) Nesting success: Proportion of eggs producing fledglings in first broods from which at least one young fledged (arc sine transformed for analyses).
(4) Fledglings/pair: number of young fledged from first broods that produced at least one fledgling.
(5) Nestling body mass: Body mass of first brood nestlings at 15 days of age, excluding nests from polygynous males.
(6) Adult mass: Body mass of adult trapped when feeding nestlings aged 8–12 days.
If competition was important we should find that laying date was delayed and/or that clutch-size, nesting success, fledglings/pair, or mass was reduced in the situation with more intense competition.
RESULTS
Blue Tit density under different nest box configurations
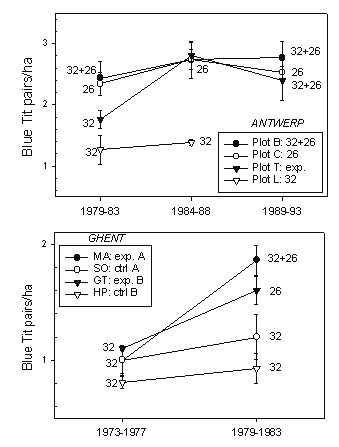
The Blue Tit densities in all periods included in this paper are shown in Fig. 1.
In Table 2 we compare between-period density changes in the control and experimental plots. To remove any effect of a change in density caused by period effects rather than by experimental treatment effects we compared the density ratios of the experimental plot in the two 5-year periods (where the nest box configuration was changed) to that in the control plot (where it was not changed). The ratio of these ratios is the treatment effect.
For example in Plot MA the post-treatment density (small and large-holed nest boxes) was 1.86 pairs/ha; the pre-treatment density (large-holed nest boxes only) was 1.00 pairs/ha. The increase caused by adding small-holed nest boxes was not times 1.86, because in the control plot breeding density increased from 1.00 to 1.20. The increase caused by the treatment was times 1.55 (1.86:1.20).
Since we expected an increase in Blue Tit breeding density when we added small-holed nest boxes, we used a one-tailed significance level for the PERIOD*PLOT interaction from the PROC MIXED analysis with PLOT, PERIOD and PLOT*PERIOD as factors. In the Plot MA and Plot SO example P = 0.017.
In all cases in which small-holed boxes were added to a study plot the mean change over a 5-year period was higher than in a control plot where the nest box configuration remained unchanged. In all cases, except one, this difference was statistically significant (Table 2).
We will use the following terminology: Blue Tit density is ‘high’ in any plot in which small-holed nest boxes are present, and ‘low’ when this is not the case. This terminology is meant to be understood in relative terms, since in plot MA, for example, the mean ‘high’ Blue tit density is 1.86 pairs/ha, whereas in plot T the mean ‘low’ Blue Tit density is 1.76 pairs/ha. This simply reflects the fact that the habitat quality in plot T (as in all Antwerp plots) is much better than in most of the Ghent plots. Great Tit density is ‘high’ in plots in which the density of large-holed boxes was high, and ‘low’ (and mostly unknown) in plots in which only small-holed nest boxes were available.
Effects of competition during the breeding season
Intra-specific competition among Blue Tits
In the experimental plot we compared a 5-year period with low Blue Tit densities (only large-holed boxes) and a 5-year period with high Blue Tit densities (both large and small-holed boxes). In the control area the situation remained unchanged. That comparison can be made at Ghent by comparing Blue Tit reproduction between plots MA (changed from large-holed to mixed) and SO (large-holed boxes in both periods), and at Antwerp by comparing plot T (large holed to mixed) and Plot B (mixed in both periods) (Table 3).
In both regions laying was relatively later in the experimental plot, when Blue Tit density was higher. Thus at Ghent in the control plot SO laying was earlier during the second 5-year Period than in the first 5-year Period, whereas it remained unchanged in the experimental plot MA. At Antwerp the period effect was highly significant, laying being much earlier during the second 5-year Period in both plots. The difference between the two periods, however, was 8 days in the control plot, compared to only 5.6 days in the experimental plot, resulting in a significant Plot*Period interaction.
At Ghent nesting success was significantly affected by intra-specific competition (Plot*Period P = 0.03), whereas at Antwerp this was not at all the case.
Body mass measures were available at Antwerp only. Fifteen-day nestling body mass was 2.6% lower when Blue Tit density was high in the experimental plot, as compared to when low Blue Tit density was low.
The adult body mass analysis yielded a significant three-way interaction term sex*plot*period (P = 0.0025), indicating that the period effect differed between plots and sexes. Hence we performed analyses separately for males and females. Male mass did not change with the intensity of competition, but female mass did decrease by about 3% in the high Blue Tit density period in the experimental plot.
In both Ghent and Antwerp we therefore find evidence for the existence of intra-specific competition. If the effect on laying date is the result of increased competition, this must be for food during the early part of the breeding season. Effects on nesting success and on body mass must be the result of increased competition for food during the nestling stage. A reduced fledgling mass could result in reduced survival after fledging (Perrins 1979).
Interspecific effects of Great Tits on Blue Tit reproduction
In the experimental plot we compared a 5-year period with low Great Tit densities (only small-holed boxes) and a 5-year period with high Great Tit densities (both large and small-holed boxes). In the control area the situation needed to remain unchanged. That comparison could only be made in the Antwerp plots where plot T (the experimental plot) had small-holed nest boxes only in the breeding season 1984–1988, and where both box types were present during the 1989–1993 season. In Plot B the treatment throughout was 32+26.
The results (Table 4) are surprising because we find a hint that Blue Tit clutch-size may be adversely affected by Great Tit numbers (as Perrins & McCleery 1989 found), but especially in that we find significant effects on nestling body mass (one-tailed P = 0.006) and on female (one-tailed P-value = 0.017) but not on male body mass. The overall analysis of adult mass in which Plot, Period and Sex were included as main effects and all sex*factor interactions were also added, yielded several significant interaction terms: sex*period (P = 0.03) and an almost significant sex*plot (P = 0.06). We therefore analysed the two sexes separately.
Summary of effects during the breeding season
For Blue Tits we tested both for a possible effect of intra- and interspecific competition, because, according to Reynoldson & Bellamy (1971) intra-specific competition must exist for interspecific competition to be possible.
In the Antwerp plots we found no significant effect of either on clutch-size, nesting success or number of young fledged per pair. We did find, however, that 15-day-nestling body mass and adult female, but not male body mass, were significantly reduced at higher densities of either species, and that laying was relatively later when Blue Tit density was experimentally increased.
In the Ghent comparison we could only study a possible effect of intra-specific competition. In this region, where the overall feeding conditions are less favourable, we found intra-specific effects on laying date and on nesting success. The latter effect was not detected at Antwerp.
All in all our results show that interspecific competition between Great and Blue Tit during the breeding season does occur. It seems to be stronger in areas/years in which food conditions are poorer.
Our results also show that interspecific competition does not occur in every year, implying that longer-term studies are more likely to detect them. This was suggested by Schoener (1983) in his review, although he underlined that very few long-term experimental studies on interspecific competition exist.
What Blue Tit population processes are affected when small-holed nest boxes are present and Blue Tit densities are higher?
When large numbers of small-holed nest boxes are provided before winter the number of Blue Tits found roosting in the nest boxes in winter increases 3–6 fold and the breeding population in the following breeding season increases by 20–60% (Dhondt et al. 1991). Aviary experiments show that, given the choice, Blue Tits prefer large-holed boxes for winter roosting, but shift to small-holed boxes when Great Tits are present in the aviary. Winter competition for roosting site, therefore, plays an important role in the life of Blue Tits. By alleviating interspecific competition for cavities through the provision of small-holed nest boxes, Blue Tits acquire access to adequate winter roosting sites, and this results in a higher breeding density.
The basic equation describing changes in numbers within populations states that numbers increase by birth and immigration, and decrease by death and emigration. If we want to understand what causes a change in the number of breeding birds in our resident populations we must re-write that equation somewhat. Gains are not from births but from recruits (first time breeders). Losses are from mortality of birds that have bred previously, and only very rarely from emigration (Dhondt & Eyckerman 1980). The breeding population thus consists of first-time breeders and birds that already bred in the study plot in a previous season (survivors). Since most birds breed in nest-boxes, and since nearly all breeding birds are marked or identified, it is possible to distinguish between these two groups.
First-time breeders can be further split into two groups: immigrant recruits (born outside the study plot, mostly of unknown origin), and local-born recruits (born in the plot in which they later breed).
If we compare the breeding population (N) in two consecutive years t–1 and t, the change in numbers is defined as
Nt / Nt-1In any one year the breeding population can be subdivided into the three groups defined above: adults that bred in the previous year (AD); local born recruits (LR); immigrant recruits (IR) or
Nt = ADt + LRt + IRtThe change between year (t–1) and year (t) is then
Nt / Nt-1 = (ADt + LRt + IRt ) / Nt-1The change in numbers is the sum of the following three rates:
(1) the adult survival rate: ADt /Nt-1 (2) the local recruitment rate: LRt / Nt-1 e.g. the number of new local-born recruits entering the breeding population per adult of the same sex in the previous years. (3) the immigration rate: Rt / Nt-1 e.g. the number of immigrant recruits joining the breeding population per adult of the same sex in the previous years.The reason we use the number of same-sex adults, rather than breeding pairs is that polygyny in Blue Tits is rather common (Dhondt 1989b), and hence the number of females is often larger than the number of males.
When we provided small-holed nest boxes Blue Tit breeding populations shift from a ‘low’ to a ‘high’ density. How does that increase come about, and how is it maintained?
In order to increase the density, at least one of the ‘rates’ must increase, but the new ‘high’ density can be maintained without any of the rates having to be different after the density increased. Of course, if the rates were the same before and after the density increase, the numbers of birds in each group will be larger. If at the new high density one rate is higher/lower than before, then one or both other rates must have decreased/increased. An implication of all this is that we need to evaluate what happens in the year of the change from low to high density separately from what happens in the subsequent high density years.
Our strategy to understand possible changes in population processes was to carry out two independent analyses: (1) To model adult survival rates using SURGE, a program that uses capture–recapture data of individually marked birds (Lebreton et al. 1993); (2) to compare the proportion among new recruits that was born locally or immigrated from elsewhere (provides information on changes in the ratio of local versus immigrant first time breeders); (3) and to compare the local recruitment rates between periods: This provides information on the locally realised fitness from the perspective of the breeding adult.
Adult survival rates
In order to model adult survival rates adequately one needs to capture a high proportion of the breeding adults in each year. Furthermore, in order to have sufficient power to distinguish between different models, sample sizes need to be large. Because one or both conditions were not met in some of the Ghent plots, the model selected by SURGE in the Ghent plots was one of constant survival in the periods studied (results not shown). If interspecific competition had an effect, we were unable to detect it.
In the Antwerp sites the populations tended to be larger, and 85% or more of the adults were trapped in each year. We feel, therefore, that survival analyses are meaningful for this data set.
Following the strategy developed by Lebreton et al. (1992), we first modelled recapture rates. These were constant, and did not vary between plot (data not shown). We then compared males and females in each plot. Within each plot the st, p model in which the survival rates of males was equal to the survival rate of females were significantly better than the model in which they differed (Table 5). Because we were interested in possible effects of competition on survival, and therefore needed to compare plots with different treatments, we combined male and female captures in each plot.
We then compared different models testing for the possible effect of the experimental manipulation on adult survival rates (Table 6).
Adult survival varied significantly between years, since the st, p-model was much better that the s, p model (constant survival over time). The st, p-model was compared to three models in which an effect of the experimental increase in Blue Tit density was assumed: (1) The survival rate differed between plot T and B only in 1984, the year of the increase in density. In all other years survival rates in Plots B and T were the same. This added one survival parameter. (2) Survival in B and T were the same for 1979–1982, but different in the other 10 years. This gave a model with 4+10+10 survival parameters. (3) Survival was different in the years 1979–1982, but the same in all later years. In this model there were 4+4+10 survival parameters.
The results presented in Table 6 show unambiguously that the model assuming equal survival rates in the two plots was the best. We therefore conclude that there is no evidence that the increase in Blue Tit population density from 1983 to 1984 would be caused by in increase in adult survival rate in Plot T compared to Plot B.
We must also conclude that throughout the high density period Blue Tit survival rate in Plot T did not increase (or decrease for that matter, in case there was density dependent survival) compared to Plot B. Interspecific competition does not influence adult survival rates in this study.
Explanations of how the population in Plot T increased, and how it remained high must, therefore, be sought from recruitment.
The proportion of local born recruits among first-time breeders
The initial analyses for plots B and T showed no difference in the proportion local born recruits between the year of density change and the subsequent years during which Blue Tit density had been experimentally increased. We therefore present only the results for a two-period comparison: all low density years and all high density years. Because our analysis yielded a significant three-way interaction (F1,36.4 = 4.07; P = 0.05), we repeated the analyses by sex. In these analyses the PLOT*PERIOD interaction was significant for males (F1,13.1 = 5.51; P = 0.035), but not for females (F1,24 = 0.08; P = 0.78) (Table 7). This analysis shows that in the Antwerp plots when Blue Tit density increases, and remains higher than in the low-density situation with only large-holed nest boxes, the local recruitment rate for males, but not for females, increases. Coincident with this increase the proportion of local born birds in the breeding population increases, and, therefore, the proportion and number of birds immigrating to the population from elsewhere decreases.
Recruitment rates
We now need to determine if the increase in the size of the breeding population is caused by more local born recruits joining the breeding population, by more immigrant recruits coming in, or because both were true.
To answer the first question we compared, for the Antwerp data, local recruitment rates between plots and sexes in three periods defined in relation to the experimental density in Plot T. To answer the second question we compared the proportion of local-born recruits among first-time breeders. If the relative contribution of birds born on the plot or immigrating from elsewhere changed, the proportion of local born birds among recruits should change, and the relative contribution of immigrant recruits should then have changed inversely.
Plots T and B in Antwerp
We grouped the breeding seasons into three periods, according to the nest box configuration in plot T: period 1: 1980–1983: low Blue Tit density; period 2: 1984: year of change to high density; period 3: 1985–1993: high density period.
We studied variation in Local Recruitment Rate (LRR) using PROC MIXED and the Macro GLIMMIX (SAS 1997) with three main effects (PLOT, PERIOD, SEX) and all interactions. YEAR was randomised within PERIOD. The three-way-interaction term was highly significant (F2,33 = 6.11, P = 0.006), indicating that the change in LRR between periods differed between plots and sexes. We therefore continued the analyses for each sex separately and found a significant PLOT*PERIOD interaction for males (F2,11 = 6.87. P = 0.01), but not for females (F2,11 = 0.11. P = 0.90). A more detailed examination of the results (Table 8) shows that the significant interaction is caused by the PERIOD1*PLOT_T term being significantly different from zero. This implies that there are no significant differences between the LRR in the year of change, and in later years.
The Ghent plots
The Ghent plots had smaller breeding populations, and a smaller proportion of breeding adults was trapped. Furthermore the plots MA and SO are smaller than the Antwerp plots so that the number of locally recruiting Blue Tits is small. We, therefore, had to combine the results for the two sexes. Since above we found no difference between the year of change from low to high density, and later high-density years, we also combined these two periods into one.
In the MA–SO comparison the PLOT*PERIOD interaction in a PROC MIXED analysis was not statistically significant for the LRR analysis (F1,8 = 2.33, P = 0.16), although the LRR increased from 0.02 to 0.11 in MA, compared to no change in plot SO (Table 9). The increase in the proportion local first time breeders in Plot MA (from 0.02 to 0.18) was significantly larger that the increase in Plot SO from 0.03 to 0.07 (F1,8 = 6.99, P = 0.03).
DISCUSSION
We were able to manipulate the breeding density of Great and Blue Tits by changing the type of nest boxes available, confirming our previous results that providing small-holed nest boxes causes Blue Tit breeding density to increase (Dhondt 1989a; Dhondt et al. 1991). This made it possible to carry out long-term manipulative experiments to determine the possible effects of intra- and interspecific competition on Blue Tit reproduction, and to determine which population parameter caused the Blue Tit breeding density to increase.
Our experimental approach was to compare two study plots during two 5-year periods. In one of the study plots we maintained the nest box configuration unchanged, whereas in the other plot we changed the nest box configuration and therefore the density of one or the other species. A significant plot by period interaction was considered evidence for the existences of either intra- or interspecific competition. We realise that an alternate approach would have been to reverse the nest box configuration in both plots. We felt that keeping the nest box configuration unchanged in the control plot provided insight in possible environmental effects that we could not control. An example of such an effect was three successive extremely cold winters in the 5-year period 1984–1988 (the winters 1984–1985, 1985–1986 and 1986–1987) resulting in very poor local recruitment in the control Plot B, compared to the previous five-year period. Another approach, still, would have been to simply correlate the annual densities of Blue and Great Tits against the variable of interest over a ten-year period (as done for example by Dhondt et al. 1992). We felt our analyses comparing mean values over five-year periods was philosophically more appropriate, because we had designed the experiments specifically to test the hypotheses described above.
Effects of intra- and interspecific competition on Blue Tit reproduction
Very little was known about the possible existence of intra-specific competition in Blue Tits. Our results show that intra-specific competition in Blue Tits during the breeding can be documented, but some of our results were surprising.
Laying date
In both replicates we observed that in high-density situations Blue Tits laid on average later compared to the control plot. It is surprising that no similar effects of competition on laying date have been reported before, because many experimental food addition experiments have shown that birds advance laying when additional food is provided. (Svensson 1995) The results of the food supplementation experiments show that just before laying food is limiting. This implies that competition might be intense at that time, and it is therefore not really surprising that we can experimentally document its existence. The reason why this has not been documented before could be that laying dates vary strongly between years. In our data, for example, we find in the Antwerp plots a period effect of about 8 days compared to an effect of intra-specific competition of only about 2 days (Table 3). Interspecific competition did not have a significant additional effect on laying date.
Clutch-size
In the present analysis we did not find an effect of Blue Tit density on Blue Tit clutch-size, although in an earlier paper (Dhondt et al. 1992) we reported such an effect in Plot T. In that study we found that Blue Tit clutch-size was inversely related to Blue Tit density over the period 1979–1988. As can be seen in Table 3 Blue Tit clutch-size in Plot T during the 5-year low-density period 1979–1983 (12.01±0.22) was smaller than in the 5-year high-density period 1989–1993 (11.13±0.20), but we observed a similar difference between the two periods in the control plot. We must therefore conclude that effects of intra-specific competition on Blue Tit clutch-size are infrequent at best. It is therefore surprising that our data suggest (P = 0.07) that Great Tit density may have an effect on Blue Tit clutch-size.
Nesting success and nestling mass
In the poorer quality Ghent study sites, but not in the better Antwerp plots, nesting success was inversely related to Blue Tit density. Although in the Antwerp plots we did not find an effect of density on nesting success, the reduction in fledgling mass of 2.6% was quite significant. This suggest that that intra-specific competition during the nestling stage operates in both regions, but is stronger in the poorer plots. The increase in Great Tit density had an effect on Blue Tit fledgling mass that was very similar in magnitude to the effect of intra-specific competition.
Adult mass
A surprising result was that female but not male mass was lower in the experimental plots as compared to the control plot both when the intensity of intra- and the intensity of interspecific competition increased. By comparing the mass of the same birds in successive years we were able to show that it was an individual adjustment, and not caused by smaller/lighter females joining the breeding population. As with brood-size manipulation experiments this result shows how males and females have different strategies as concerns their investment in reproduction (Nur 1988).
When comparing the effects of intra- and interspecific competition on Blue Tit reproduction we find that they seem to operate to some extent at different times in the breeding season. Thus in the pre-laying period the effect of intra-specific competition is strong, but interspecific competition has no impact. This suggests only limited overlap in foraging niches. During the laying period either effects are weak, although our data suggest that perhaps interspecific competition might have a larger impact. During the nestling stage both Blue and Great Tit density of seem to have a similar impact on nestling mass and on female body mass. In that period both species mainly forage on caterpillars, and overlap extensively in food brought to the nestlings (Nour et al. 1998).
Effects of interspecific competition on population rates
Adult survival rates did not change in the experimental Plot T compared to the control Plot B when the intensity of intra- and/or interspecific competition were varied. This result is even more surprising because female body mass was lower in the final 5-year period, suggesting that they had to work harder at raising their young. The increase in breeding density, therefore, was not caused by increased adult survival rates.
Both at Ghent and Antwerp the rate which did change, and hence caused the increased density was the local recruitment rate. More local born young per breeding adult remained in their birth plot in the year of the population increase but also in later years. This implies that when small-holed nest boxes are available the quality of the plot improves, resulting in more local-born birds remaining in their birth plot thereby causing an increase in breeding density. In the Ghent plots the local recruitment rate increased for both sexes, in the Antwerp Plots they increased only for the males.
The percentage local born young also increased in periods of increased density, implying that the immigration rate must have decreased, compared to the low-density period. The composition of the population, as regards its origin, therefore changed. At higher experimental densities a higher proportion of the male breeding birds were born locally.
Roosting behaviour in the winter can explain the increased local recruitment rates. In the fall, long before tits start using nest boxes (or other cavities) for roosting, the males set up autumn territories. Adults already defend autumn territories when still moulting, and song is frequently heard at that time (Dhondt 1973). Adults usually re-occupy the same territory that they occupied in the previous breeding season (Dhondt 1971). While wandering around, juveniles frequently become involved in skirmishes. Drent (1983) described this behaviour in great detail for Great Tits. What is important in that context is that the ability to win skirmishes plays an important role in the decision of a juvenile to stay in an area (Drent 1983). During autumn, tits can frequently be observed to inspect cavities, and although they do not use them at that time, cavities are actively defended. The presence of adequate nest boxes influences the decision to remain in an area (Drent 1987). Blue Tits that inspect large-holed nest boxes claimed by Great Tits are repeatedly chased away when inspecting them. Later, when the leaves have fallen, and tits roost in cavities during cold winter nights, very few Blue Tits will be found in large-holed nest boxes. An area without small-holed nest boxes (or other cavities that cannot be used by the larger Great Tit) is therefore not very attractive for Blue Tits, and most of the birds born in the area will leave it before winter.
By providing small-holed nest boxes before winter, we improve the quality of an area as a wintering site for Blue Tits. Drent (1983) showed that tits roosting in boxes were less likely to be found in owl pellets. Roosting in a safe cavity thus has survival value. We can speculate that young Blue Tits, as they search for an adequate wintering area, might be more inclined to stay close to the area in which they were born, if they find adequate roosting sites there. That this would mainly influence the males is not surprising, since males are more frequently found in nest boxes in winter (Dhondt et al. 1991).
CONCLUSIONS
Although adverse effects of intra- and interspecific competition during the breeding have been demonstrated on Great Tits (review in Dhondt 1989), very little was known about possible adverse effects of increased densities of their own or other species on Blue Tit reproduction. Our experiments show that increases in the breeding density of con- and hetero-specifics have some negative impact on Blue Tit reproductive success. At higher densities of competitors the birds lay later, raise fewer young per egg laid in the poorer study plots, and fledge lighter young. At higher densities of competitors female, but not male breeding-season body mass is lower, suggesting that females also suffer from competitive interactions. In our study we did not find that this would affect their subsequent survival, but our results are in line with results obtained by Nur (1988). He found that when he manipulated clutch-size in Blue Tits, females but not males would pay a survival cost. The effects were clearer in some years than others, suggesting that adverse effects of competition on the survival of breeding females might occur in certain years and/or areas.
The provision of small-holed nest boxes increased the breeding density of Blue Tits in all cases. This most probably resulted directly from these small-holed boxes, which offered safe roosting sites during winter, improving habitat quality for wintering birds. More Blue Tits used nest boxes for roosting when Great Tits could not use them or were not present in aviaries (Kempenaers & Dhondt 1991). Dispersal out of the natal plot, especially by the young males was much reduced. This suggests that juvenile birds are less likely to disperse out of high quality habitat regardless of the cause of the high quality.
At the same time relatively fewer birds born outside the study plot immigrated to the plots which contained small-holed nest boxes suggesting that competition for these high quality areas must have been intense.
Surprisingly adult survival did not change with changes in competition. One could have expected that when safe winter roosting sites were available, and adults more frequently used them, this might have increased the changes of adult survival. Conversely, one might have expected that at higher breeding density, adult survival rates could have decreased. Our results suggest neither change happened.
Our experiments complicate somewhat the dichotomy from Wiens’s (1989) review. He separated experimental manipulations of resources from experimental manipulations of density. In our case we have manipulated resources in order to influence density. In field experiments on competition manipulation of one (resources) should result in a change in the other one (density), if the experiments are carried out over a long enough period.
ACKNOWLEDGEMENTS
This research was supported by several grants of the Belgian Fund for Scientific research. The data at Ghent were collected mainly by Jan Hublé, Raymond De Waele, Prosper Bekaert, André Dhondt, Roman Eyckerman and Jenny De Laet. The data at Antwerp were mainly collected by André Dhondt, Frans Fierens, Janine Schillemans, Jenny De Laet, Frank Adriaensen, Erik Matthysen, Werner Plompen. Many others also participated in the fieldwork. Andy Gosler and Rauno Alatalo constructively commented on the manuscript.
REFERENCES
Bock, C.E., Cruz, A. Jr., Grant, M.C., Aid, C.S. & Strong, T.R. 1992. Field experimental evidence for diffuse competition among southwestern riparian birds. American Naturalist 140: 815–828.
Connell, J.H. 1980. Diversity and coevolution of competitors, or ghost of the competition past. Oikos 35: 131–138
Connell, J.H. 1983. On the prevalence and relative importance of interspecific competition: evidence from field experiments. American Naturalist 122: 661–696.
Dhondt, A. A. 1971. Some factors influencing territory in the Great Tit Parus major L. Giervalk 61: 125-135.
Dhondt, A.A. 1973. Postjuvenile and postnuptial moult in a Belgian population of Great Tits, Parus major, with some data on captive birds. Giervalk 63: 187-210.
Dhondt, A.A. 1989a. Ecological and evolutionary effects of interspecific competition in tits, Parus spp.. The Wilson Bulletin 101: 198-216.
Dhondt, A.A. 1989b. Blue Tit. In: Newton, I. (ed.) Lifetime Reproduction in Birds. Academic Press: 15–33.
Dhondt, A.A. & Eyckerman, R. 1980a. Competition between Great Tit and the Blue Tit outside the breeding season in field experiments. Ecology 61: 291-1296.
Dhondt, A.A. & Eyckerman, R. 1980b. Competition and the regulation of numbers in Great and Blue Tit. Ardea 68: 121- 132.
Dhondt, A.A., Eyckerman, R., Moermans, R. & Hublé, J. 1984. Habitat and laying date in Great and Blue Tit. Ibis 126: 388-397.
Dhondt, A.A., Kempenaers, B. & Adriaensen, F. 1992. Density-dependent clutch size caused by habitat heterogeneity. Journal of Animal Ecology 61: 643–648.
Dhondt, A.A., Kempenaers, B. & De Laet, J. 1991. Protected winter roosting sites as a limiting resource for Blue Tits. Acta XX Congressus Internationalis Ornithologici: 1436–1443.
Dhondt, A.A., Schillemans, J. & De Laet, J. 1982. Blue Tit territories at different density levels. Ardea 70: 185–188.
Drent, P.J. 1983. The functional ethology of the Great Tit Parus major L. Ph.D. thesis University of Groningen.
Drent, P.J. 1984. Mortality and dispersal in summer and its consequences for the density of Great Tits Parus major at the onset of autumn. Ardea 75: 59–72.
Drent, P.J. 1987. The importance of nestboxes for territory settlement, survival and density of the Great Tit. Ardea 75: 59–72.
Hairston N.G. 1980. The experimental test of an analysis of field distributions: competition in terrestrial salamanders. Ecology 61: 817–826.
Hogstad, O. 1975. Quantitative relations between hole-nesting and open-nesting species within a passerine breeding community. Norwegian Journal of Zoology 23: 261–267.
Keddy, P.A. 1989. Competition. Chapman and Hall, London New York.
Kempenaers, B. & Dhondt, A.A. 1991. Competition between Blue and Great tit for roosting sites in winter: an aviary experiment. Ornis Scandinavica 22: 73–75.
Lebreton, J.D., Burnham, K.P., Clobert, J. & Anderson, D.R. 1992. Modeling survival and testing biological hypotheses using marked animals – a unified approach with case-studies. Ecological Monographs 62: 67–118.
Lebreton, J.D., Pradel, R. & Clobert, J. 1993. The statistical analysis of survival in animal populations. Trends in Ecology and Evolution 8: 91–95.
Loeb, S.C. & Hooper, R.G. 1997. An experimental test of interspecific competition for Red-Cockaded woodpecker cavities. Journal of Wildlife Management. 61: 1268–1280.
Lundberg, A. & Alatalo, R.V. 1992. The Pied Flycatcher. T & A.D. Poyser, London.
Minot, E.O. &. Perrins, C.M. 1986. Interspecific interference competition – nest sites for Blue and Great Tits. Journal of Animal Ecology 55: 331–350.
Monkkonen, M., Helle, P. & Soppela, K. 1990. Numerical and behavioural responses of migrant passerines to experimental manipulation of resident tits Parus spp.: Heterospecific attraction in northern breeding bird communities. Oecologia 85: 218–225.
Nour, N., Currie, D., Matthysen, E., Van Damme, R. & Dhondt, A.A. 1998. Effects of habitat fragmentation on provisioning rates, diet and breeding success in two species of tit (great tit and blue tit). Oecologia (Berlin) 114: 522–530.
Nur, N. 1988. The consequences of brood size for breeding Blue tits III. Measuring the cost of reproduction survival future fecundity and differential dispersal. Evolution 42: 351–362.
Perrins, C.M. 1979. British Tits. Collins, London.
Perrins, C.M. 1990. Factors affecting clutch-size in Great and Blue Tits. In: Blondel, J. et al (eds) NATO ASI Series, Vol. G 24; Population Biology of Passerine Birds. Springer-Verlag Berlin Heidelberg: 121–130
Pimentel, D., Feinsberg, E.H., Wood, P.W. & Hages, J.T. 1965. Selection, spatial distribution and the evolution of competing fly species. American Naturalist 99: 97–109.
Reynoldson, T.B. & Bellamy, L.S. 1971. The establishment of interspecific competition in field populations with an example of competition in action between Polycelis nigra (Mull) and P. tenuis (Ijima) (Turbellaria, Tricladida). In: Den Boer, J. & Gradwell, G.R. (eds.) Proceedings of the Advanced Study Institute on ‘Dynamics of Numbers in Populations’ Oosterbeek 1970, PUDOC, Wageningen: 532–547.
SAS. 1997. SAS/STAT Software: changes and enhancements through Release 6.12. SAS Institute, Cary, North Carolina.
Schoener, T.W. 1983. Field experiments on interspecific competition. American Naturalist 122: 240–285.
Svensson, E. 1995. Avian reproductive timing – when should parents be prudent. Animal Behaviour 49: 1569–1575.
Van Balen, J.H. & Potting, R.P.J. 1990. Comparative reproductive biology of four Blue Tit populations in the Netherlands. In: Blondel J. et al. (eds) NATO ASI Series, Vol. G 24; Population Biology of Passerine Birds, Springer-Verlag Berlin Heidelberg: 19–38.
Wiens, J.A. 1989. The ecology of Bird communities. Vol 2; Processes and variations. Cambridge University Press, Cambridge.
Table 1. Experimental nest box configuration and shorthand notation used in text.

Table 2. Comparison of the change in mean Blue Tit breeding densities in experimental versus control plots, under different nest box configurations, and one-tailed P-value testing the significance of the plot*period interaction term under a Proc Mixed analysis.

Table 3. Mean ±SE of various reproductive traits in Blue Tit populations experimentally subjected to different intensities of intra-specific competition. The experimental plots are MA and T; the control plots are SO and B. P-values are two-tailed for main effects, and one-tailed for the plot*period interaction term. At Ghent n = 203 for laying date and clutch-size; n = 173 for the other variables. At Antwerp n = 309 for male body mass; n = 359 for female body mass; n = 448 for the other variables. Significance of nesting success calculated on arcsine transformed values.
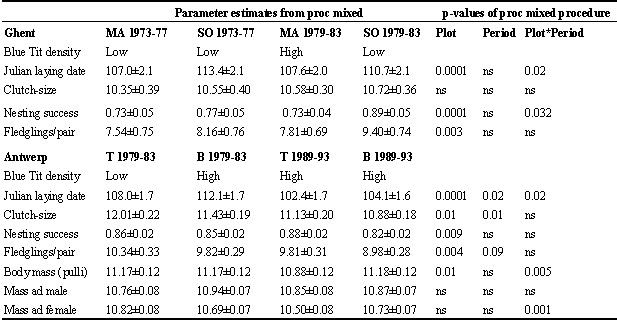
Table 4. Mean ±SE of various reproductive traits in Blue Tit populations experimentally subjected to different intensities of interspecific competition. P-values are two-tailed for main effects, and one-tailed for the plot*period interaction term. At Antwerp n = 397 for female body mass; n = 348 for male body mass; n = 500 for the other variables. Significance of nesting success calculated on arcsine transformed values P-values are two-tailed for plot and for period, but one-tailed for the plot*period interaction term, because we expect that reproduction in plot T will be less successful in the period with a high Great tit density (1989–1993).
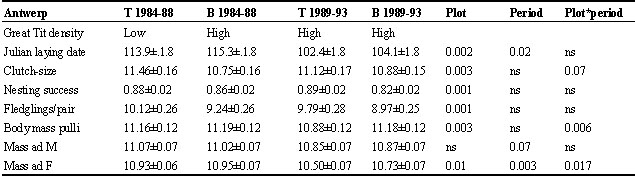
Table 5. Aikake Information Criterion (AIC) values for st, p-models in each plot comparing the case in which male and female survival rates were equal or different. In brackets the number of parameters estimated. A model for which the AIC value is more than 2 units smaller is significantly better (Lebreton et al. 1992).

Table 6. Table results from SURGE models testing a possible effect of interspecific competition on adult survival rates – Comparisons of Pots T and B, both sexes combined. In all cases the recapture rate was constant, and equal in Plots T and B. T = B means that survival rates were assumed to be equal in the two plots.
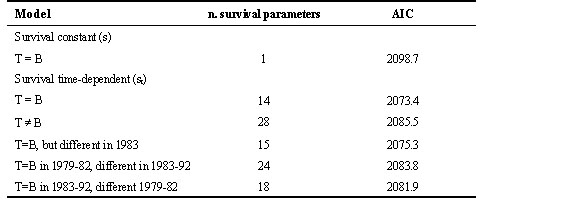
Table 7. Proportion of local recruits (arcsine transformed) among first-time breeders in the Antwerp study plots (Means ±SE). Pre: pre-experimental years; change: first high-density year in Plot T; post: other high-density years in Plot T. Note that in Plot B Blue Tit density was high throughout.
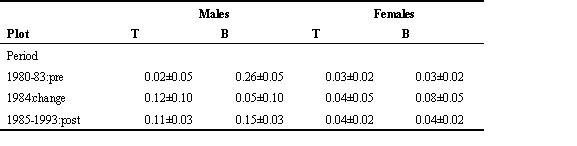
Table 8. Local Recruitment Rates in Antwerp (Means ± SE) (see Table 7 for more details).
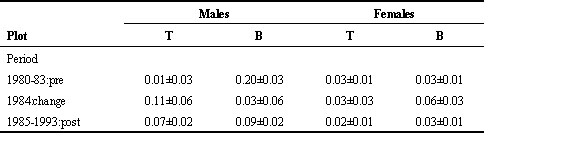
Table 9. Mean ±SE for local recruitment rate and proportion local born recruits in two plots at Ghent (males and females combined) (see Table 7 for more details). In Plot SO Blue Tit density remained low throughout; in Plot MA Blue Tit density was low in the period 1973–1977, and high in the period 1979–1983.
Fig. 1. Mean Blue Tit breeding density per 5-year period in different nest box configurations at Antwerp (above) and Ghent (below). In all cases the presence of small-holed boxes (26 mm) resulted in an increase in breeding density as compared to control plots. Values are 5-year means ±SE. The statistical comparisons are made in Table 2 where more details on the nest box configurations are given.