Plenary06: Taxon cycles in the Lesser Antillean avifauna
R.E. Ricklefs1,2 & E. Bermingham2
1Department of Biology, University of Missouri-St Louis, 8001 Natural Bridge Road, St Louis, MO 63121-4499,USA, e-mail ricklefs@jinx.umsl.edu; 2Smithsonian Tropical Research Institute, PO Box 2072, Balboa, Republic of Panama, USA (from the US, Unit 0948, APO AA 34002-0948), e-mail eb@naos.si.edu
Ricklefs, R.E. & Bermingham, E. 1999. Taxon cycles in the Lesser Antillean avifauna. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban. Ostrich 70 (1): 49–59.
Patterns of taxonomic differentiation and geographical distribution of West Indian birds suggest that taxa may pass through phases of increasing and decreasing geographical range. Such sequences are referred to as taxon cycles. Although decline may often lead to extinction, new phases of expansion, accompanied by increased or shifted habitat distribution are also possible. The taxon cycle is a historical hypothesis, but its existence was formerly inferred from contemporary, indirect evidence. Molecular studies of land birds in the West Indies now provide relative ages for taxa based on genetic differentiation among island populations. These age estimates confirm that older populations tend to have restricted geographical and ecological distributions. That ecology and geography are strongly correlated with age across taxa also suggests that the time course for evolutionary change through the taxon cycle is relatively consistent among independently evolving populations. Because young taxa have continuous distributions within the West Indies, gaps in the ranges of older taxa indicate extinctions of island populations. Starting from this premise, one may estimate exponential extinction rates of about 50% per million years for island populations in the Lesser Antilles, which indicates an average population life span of two million years. The relative timing of expansion phases suggests that cyclic dynamics of populations are not driven by extrinsic factors such as climate and associated habitat change during glacial cycles. Alternatively, such cycles may be intrinsic, perhaps driven by lags in the evolutionary responses of host and parasite populations. This is suggested by observed variation in the prevalence of blood parasites among island populations of the same species. The taxon cycle concept provides a useful paradigm for understanding variation among species in geographical range, ecological distribution, and vulnerability to extinction.
INTRODUCTION
Some ideas are so attractive that they surface repeatedly in spite of having been considered and rejected by earlier generations of biologists. One such idea is that the organism provides a useful analogy for understanding the functioning of larger biological systems, including populations, evolutionary lineages, and ecological communities. For example, many biologists have entertained the notion that evolutionary lineages, whether populations or large monophyletic groups, progress through a series of life stages much as the individual progresses from youth to maturity to old age. This idea was more acceptable 50 years ago than it is now. In a chapter on ‘Species Senescence’ in his book Foundations of Plant Geography (1944), the botanist S.A. Cain began: ‘The concept that certain species are senescent is fairly widespread in taxonomy and plant geography. Populations of this sort are variously referred to as old, weak, conservative, and unaggressive, whereas other species are characterised as young, strong, competitive, dominating, and vigorous.’ Species, often closely related species, can differ greatly in their distribution and abundance; the study of these differences is part of what is now called ‘macroecology’ (Brown & Maurer 1989; Brown 1995). It is tempting to conceptualise patterns of distribution and abundance as stages in the life history of a taxon and to compare the contraction of a population to senescence.
The idea of species senescence has not fared well. Clearly, organism analogies applied to larger biological systems are inappropriate in many ways. With regard to the issue of species senescence, unlike organisms, populations continually rejuvenate themselves by means of the death and replacement of individuals. Also, unlike organisms, populations have no developmentally fixed ‘soma’, equivalent to our bodies, to undergo intrinsic physiological decline (Rose 1991). Indeed, our preoccupation with climate change, habitat destruction, and anthropogenic extinction leads us to view species and populations as being at the mercy of the environment. For the most part, ecologists believe that changes are imposed on populations from outside.
Nonetheless, we suggest in this contribution that the organism analogy contains a kernel of truth and that it offers alternative ways of regarding changes in populations over time and space. The birth of an individual is associated with its development from a single cell, or a small number of cells in the case of budding and vegetative reproduction. The birth of a new lineage has been related to similarly formative genetic events. One model of such a process is peripatric speciation, by which new species are thought to arise through the formation of small, peripheral isolates (Mayr 1963; Bush 1975). This process has been associated with such birth traumas as ‘genetic revolution’ (Mayr 1963; Gould & Eldredge 1977) and ‘punctuated evolution’ (Eldredge & Gould 1972; Stanley 1979). In each case, the ‘birth’ of a population is accompanied by substantial genetic change.
By definition, aging requires a soma, what our genetic lineage uses to make more of itself. The soma ages because it accumulates damage that can’t be repaired and this damage eventually leads to death (Finch 1990). Accordingly, how can a population be considered as having a soma? Individuals continually die and are born, renewing the physical presence of a population. What does not change so quickly, however, is the gene pool of the population. Evolutionary responses are relatively slow under any circumstance, requiring the selective deaths of many population’s worth of individuals to fully replace an allele (Haldane 1957). Further inertia is built into the genetic system through the infrequency of beneficial mutations and through stabilising interactions among genes, so-called ‘co-adaptation’ (Hartl & Clark 1997). Confronting this inertia is a rapidly changing environment, not so much in the sense of physical change, but in the evolving genetic strategies of enemies and other antagonists (Van Valen 1973; Hamilton 1991). Whereas a spontaneous beneficial mutation may lead to population expansion and the birth of new populations, the gene pool is otherwise relatively stagnant and begins to accumulate a counter-evolutionary burden imposed by other evolving lineages. This may lead to extinction unless some further genetic innovation should appear.
THE TAXON CYCLE CONCEPT
This makes for a nice story, but does it bear any relation to nature? Extinction itself is difficult to study because of its transient nature. To assess changes in populations with respect to age, one should compare younger and older populations. The key is being able to estimate age. A further critical assumption is that any course of change is homogeneous enough among taxa for age to have generalized biological meaning and appear as a consistent statistical effect.
In 1972, R.E. Ricklefs and G.W. Cox published a paper entitled ‘Taxon cycles in the West Indian avifauna.’ In this paper, they suggested that relative ages could be assigned to taxa on the basis of geographic distribution and taxonomic differentiation among island populations. Having established a relative age scale, they went on to demonstrate that with increasing age populations exhibited restricted habitat distribution and increased probability of extinction. A brief recounting of the logic of the taxon cycle follows. We shall restrict this discussion to the Lesser Antilles because recent work described below has focused on this region. First, a bit about the islands.
The Lesser Antilles consist of an arc of high islands of volcanic origin, stretching from the northern coast of South America north to the Greater Antilles (Donnelly 1989). Outside the volcanic arc are several low-lying islands composed of uplifted marine sediments. The ages of the islands have not been established definitively, but lava has been dated to more than 20 million years, several times older than the contemporary avifauna. Before the Lesser Antilles emerged, an older chain of islands occurred to the west, now represented by the submarine Aves Ridge. Most of the islands are separated by deep-water channels and probably have never had land connections, but the neighbours of each island are often visible from its shores. Although the climate of the Lesser Antilles is mild and the vegetation is tropical, the region is regularly traversed by hurricanes, some of gigantic proportions, and at least four of the islands have experienced volcanic activity in this century, including the currently active volcano on Montserrat (Montserrat Volcano Observatory Team 1997). Climates during glacial periods presumably were cooler and drier (Hooghiemstra 1989; Bush et al. 1990), although the effect of Pleistocene glacial cycles on the vegetation of the Lesser Antilles has not been studied.
Species in the Lesser Antilles show characteristic patterns of geographical distribution and taxonomic, generally subspecific, differentiation (Fig. 1). Subspecies distinctions are based mostly on size and plumage characteristics. In many cases, the differences between island populations are striking. The Gray Kingbird Tyrannus dominicensis is representative of species that are widespread and undifferentiated within the Lesser Antilles. The endemic Lesser Antillean Bullfinch Loxigilla noctis is equally widespread in the Lesser Antilles, but has several well-marked subspecies. Island populations of the House Wren Troglodytes aedon and Adelaide’s Warbler Dendroica adelaidae are also taxonomically differentiated; these species additionally have restricted distributions within the Lesser Antilles. At least two populations of the House Wren have disappeared during this century (Bond 1956), probably the result of predation by the introduced mongoose, and this has created a gap in the species distribution at Martinique. At the extreme, several taxa, such as the St Lucia Black Finch Melanospiza richardsoni and Whistling Warbler Catheropeza bishopi, occur only on a single island.
Ricklefs & Cox (1972) placed each Antillean species in a category defined only by its distribution and taxonomic differentiation (Table 1). From the concept of the taxon cycle developed by E.O. Wilson (1961), it was apparent that these patterns of distribution and taxonomic differentiation could be arranged in a temporal series of taxon-cycle stages. The logic of the temporal sequence is as follows (Fig. 2). Because no undifferentiated taxa have gaps in their distributions, stage 3 can only have been derived from stage 1 by differentiation (stage 2) followed by extinction, and not by haphazard long-distance colonisation. Because parallel evolution of island populations is unlikely, stage 1 species can only have originated by rapid colonisation (relative to differentiation) from a single source. Of course, taxa could remain in stage 1 indefinitely through continued migration between islands. However, it is unlikely that Antillean endemic stage 1 species might have been maintained by gene flow over long periods but nonetheless have differentiated from continental ancestors.
Wilson coined the term ‘taxon cycle’ in 1961, based on observations of Melanesian ants. A key part of his idea involved a shift from marginal to interior habitats as the cycle progressed. Wilson believed that this movement might be driven in part by competition and that declining taxa could reinitiate expansion phases. Movement of a species through the taxon cycle reflected range expansion, evolutionary differentiation between island populations, and local extinction of island populations creating gaps within the distribution. Eventually, taxa either went extinct globally or underwent a new phase of range expansion, starting the cycle over again.
If the stages of the taxon cycle represented a temporal sequence from young to old taxa, we could now begin to investigate whether populations changed with age. To this end, George Cox went to the field to quantify the abundance and habitat distributions of birds on the islands of Jamaica, St Kitts, and St Lucia, as well as in continental areas in Trinidad and Panama (Cox & Ricklefs 1977). These data showed that species in later stages of the taxon cycle tended to have reduced habitat breadth and local population density compared to species in earlier stages (Ricklefs & Cox 1978). This suggested increasing specialisation, reduced competitive ability, and perhaps increasing vulnerability to extinction with increasing age. The last possibility is shown dramatically by the proportion of historically extinct or currently threatened island populations of species belonging to each stage in the taxon cycle (Fig. 3). The birds of the Galápagos and Hawaiian Islands have suffered much more at the hand of man than those of the West Indies, but all three groups of islands exhibit a dramatic increase in vulnerability of stage 3 and 4 species compared to stage 1 and 2 species.
Ricklefs and Cox also proposed a mechanism of evolutionary counter-adaptation to drive the taxon cycle. Accordingly, species might initiate a phase of expansion after the appearance of genetic mutations that enabled them to escape enemies or otherwise greatly increase population productivity, which would promote dispersal of individuals. Accordingly, the likelihood of successful invasion is closely tied to the productivity of the source population and is less dependent on the ecological fit of the colonisers with the island environment. The success of a new colonist might even be enhanced by pathogens that accompany its invasion of an island, and which become virulent in resident populations of competitors. We refer to this as the ‘conquistador effect.’ With time, however, the biota of an island, including endemic pathogens, evolves to exploit these new successful colonists, driving down their productivity and reducing their competitive ability, causing ecological decline with age. Finally, Ricklefs and Cox suggested that taxon cycles are universal but most readily apparent in island archipelagos. It is important that the size and distances of islands be tuned to the appropriate scales of dispersal and extinction in order to produce the patterns recognisable as taxon cycle stages.
It is an understatement to say that these ideas were not greeted with universal enthusiasm and critical acclaim. There has been considerable resistance to the taxon cycle concept on each of the points mentioned above. This has centred around questions of age, phylogeny, taxonomy, and biogeography, on one hand, and the role of evolutionary responses in determining ecological relationships, on the other (Abbott 1980; Williamson 1981; Liebherr & Hajek 1990). E.C. Pielou (1979) felt that patterns of distribution could be readily explained by external factors acting on short time scales: ‘. . . the whole taxon cycle may simply be the effect of sporadically occurring climatic ‘bad years’ on species-populations too isolated for losses to be quickly made good from nearby populations. It is noteworthy that the cycle has been observed in birds and insects, flying and wind-dispersed animals that can be forced into stage I of the cycle by an exceptional storm.’ Pregill and Olson (1981) questioned the underlying mechanism of counteradaptation and instead invoked climate change as a driving force: ‘Ecological doctrine and good sense revolt at the idea that a species with a long history of adaptation to a particular environment would be at a competitive disadvantage with newly arriving colonists . . . . The concept of "counter-adaptation" is an artificial construct needed to explain a nonexistent phenomenon – the taxon cycle. The patterns of distribution that constitute the "stages" of the taxon cycle are more reasonably interpreted in terms of the effects that the cycling of habitats had on species during the alternate wet and dry periods of the Pleistocene, in combination with the varying dispersal abilities of the individual species.’ The fundamental issue is this: Does a population have a meaningful history, or is its ecology basically in equilibrium with its contemporary world?
Not all reactions to the taxon-cycle concept have been negative. For example, Lawton et al. (1994) entertained the possibility that population dynamics might have an evolutionary component: ‘Population density and size of geographic range are usually thought of as attributes determined by processes operating in ecological time; that is, we expect them to be dynamic and variable over time periods of, say, 10–100 years. But there are also poorly understood, intriguing hints of effects operating in evolutionary time. They suggest that both range and abundance are evolved, persistent, species’ characteristics. . . . One group of studies centres on the "taxon cycle" for birds on West Indian islands (Ricklefs & Cox 1978; Ricklefs 1989). Among passerines, putatively older taxa occur on fewer islands, have more restricted habitat distributions and tend to have reduced population densities. (It would be intriguing to revisit these analyses using independently derived, molecular criteria for species ages.)’
MOLECULAR PHYLOGEOGRAPHY OF LESSER ANTILLEAN BIRDS
To get a better handle on the taxon cycle, it was clearly necessary to estimate the ages of populations directly. Only in this way could we ascertain the relative ages of island populations, provide finer resolution than four coarse taxon cycle stages, and place the history of island birds in the context of geology and past climate change. To this end, since 1989 we have been engaged in a broad study of the molecular phylogenetics of West Indian birds (Seutin et al. 1993; Seutin et al. 1994; Bermingham et al. 1996; Ricklefs & Bermingham 1997; Lovette et al. 1998; Lovette et al. 1999b). We have visited most of the major islands of the West Indies and accumulated blood and tissue samples non-destructively from over 3000 individual birds, working with all the species that fly into mist nets. We won’t say much about the molecular methods or the analysis of data, as these are rather well standardised now. We are still in the midst of accumulating sequences and the analyses shown here are preliminary. Most of the genetic distances are based on the ATPase 6 and ATPase 8 genes of the mitochondrial DNA. Relative rate tests within species have failed to detect rate heterogeneity among mtDNA lineages. Where we estimate times of divergence from genetic distances, we assume a molecular clock ticking at a rate of approximately 2% sequence divergence per million years (Shields & Wilson 1987; Bermingham et al. 1992; Tarr & Fleischer 1993; Tarr & Fleischer 1995; Klicka & Zink 1997; Fleischer et al. 1998). For the most part, in species with well-differentiated mtDNA lineages, individual island populations are monophyletic. One exception to this generalisation comes from Nedra Klein’s study of Yellow Warblers Dendroica petechia (Klein & Brown 1994), where populations on some of the Lesser Antilles exhibit highly divergent lineages derived independently by colonisation from South America and the Greater Antilles.
We have found that phylogeny and geography are highly correlated among conspecific or congeneric avian populations of the West Indies. For example, the distribution of lineages of the Bananaquit (Fig. 4) indicates that Lesser Antillean birds were derived from Greater Antillean stock, and that populations on the southern Lesser Antilles (Grenada and St Vincent) are differentiated from those to the north. Populations from Guadeloupe north to the Virgin Islands and St Croix carry a single mitochondrial haplotype from the variety of haplotypes occurring on Dominica, Martinique, and St Lucia, suggesting a recent spread of the taxon through these islands with a founder event between Dominica and Guadeloupe. The root of the Bananaquit Coereba flaveola phylogeny, at about 1.5 million years, is in the Greater Antilles and Bahamas.
We used genetic distances to estimate the relative timing of two events for each taxon: (1) the beginning of the expansion or phase of expansions that created the present-day island populations in the Lesser Antilles and (2) the initial colonisation of the Lesser Antilles (Fig. 5). In the case of recent invaders from South America or the Greater Antilles, the two points are coincident. In the other extreme case of single-island endemics within the Lesser Antilles, the two points are also the same, although evidence of a secondary expansion phase within the islands may have been erased by extinction. For Antillean endemics, estimating the timing of colonisation requires identification of the continental sister taxon, and presumes that this taxon is extant. At present, we have preliminary estimates of the timing of expansion phases for 25 taxa and of colonisation times for 15 taxa out of a total of 56 species of non-raptorial land birds in the Lesser Antilles.
The relative timing of expansion phases shows that the taxon cycle stages originally designated by Ricklefs and Cox are closely related to genetic distance (Fig. 6). Thus, the sequence from stage 1 to stage 4 represents a chronology of taxon age. Because stage 2 and stage 1 species have similar genetic distances, taxonomic differentiation appears relatively quickly in the Lesser Antilles. Dispersal of individuals sufficient for colonisation apparently does not often impede genetic differentiation. The average genetic distance for stage 2 species is about 1.1%, suggesting an average age of about half a million years. Therefore, differentiation occurs rapidly only by comparison with extinction. The average genetic distance for stage 3 species, for which distribution gaps indicate at least one extinction event, is about 4.5%, indicating an average age of over 2 million years, well before the beginning of the Pleistocene. Although we have analysed few stage 4 species, the oldest appear to have colonised the Lesser Antilles as long as 5 million years ago, at the close of the Miocene.
ECOLOGICAL DISTRIBUTIONS OF LESSER ANTILLEAN BIRDS
Returning to the relationship between ecology and age, local abundance and habitat distributions have been estimated by point counts for birds on four islands within the Lesser Antilles, and for Trinidad and Tobago. The fieldwork was done, using the same methods, by George Cox (Cox & Ricklefs 1977), Joe Wunderle (1985), and Irby Lovette (unpublished data). The seven habitats examined form an environmental gradient from open grasslands (habitat position = 1) to high elevation cloud forest (habitat position = 7). For a given species, it is possible to quantify its average habitat type (habitat position), the number of habitats occupied per island (habitat diversity), and its abundance in each habitat (density) (Fig. 7). We can show that birds perceive the habitat gradient the same way we do by ordinating the habitats with respect to the distribution of bird species among them.
Habitat distributions shown in Fig. 7 are representative. The Black-faced Grassquit Tiaris bicolor is a stage 1 continental species that inhabits primarily open, lowland vegetation; the Lesser Antillean Bullfinch is a stage 2 Antillean endemic with a broad habitat range; and the Trembler Cinclocerthia ruficauda is a stage 3 Antillean endemic that is more restricted to deep forests and montane habitats. When habitat scores are averaged within each taxon cycle stage for the four Lesser Antillean islands that were censused, it is clear that progression through the taxon cycle is accompanied by reduced habitat breadth, movement from open to deep forest habitats, and to a lesser extent decrease in local abundance (Fig. 8). Thus, the essential features of the taxon cycle described by Ricklefs & Cox appear to be correct.
DISTRIBUTIONAL GROUPS OF LESSER ANTILLEAN BIRDS
Taxon cycle stages, as we have defined them, depend on subjective assessment of taxonomic differentiation. They also do not distinguish allochthonous and endemic Antillean taxa, which exhibit strikingly different distributions in the Lesser Antilles. To continue the analysis further, and particularly to investigate the role of extinction in moulding species distributions, we have reclassified species into four groups based on endemism and geographical extent within the Lesser Antilles (Table 2). Examples of these distributions are shown in Fig. 9. Group A species are continental or Greater Antillean species that are widely distributed throughout the Lesser Antilles. Group B species are continental or Greater Antillean species with limited Lesser Antillean distributions, such as the Bare-eyed Thrush Turdus nudigenis. Most of these species appear to be restricted by colonisation ability rather than extinction of established populations. In the case of the bare-eyed thrush we know this because the species has colonised the Antilles only during this century. Group C species are endemic to the Lesser Antilles but are widely distributed within the island chain. Group D species are also endemic forms but have restricted distributions, in many cases with gaps between island populations. We assume that the limited distributions of Group D species result from both restricted colonisation and extinction.
From the distribution of species through the Lesser Antilles arranged according to group (Fig. 10), one can see that group A and C species, the recently expanded taxa, are uniformly widespread. Group B species are concentrated in the southern part of the island chain closer to the colonising source in South America; a few of the group B species are Greater Antillean in origin. Presumably group B species have expanded into the Lesser Antilles recently. Group D species are concentrated on the larger core islands of the Lesser Antilles, from St Lucia north to Guadeloupe.
The age distributions of group A, B, C, and D species (Fig. 11) show that taxa with extensive distributions (mostly taxon cycle stage 1 and 2 species) are young and that Antillean endemics with restricted distributions (mostly stage 3 and 4 species) are relatively much older. The fact that restricted group B species are also young supports the notion that colonisation rather than extinction has limited their distributions. The relative times of colonisation of the Lesser Antilles by what are now group C species are similar to those of group D species. This is consistent with the idea that group C species represent secondary expansions of formerly restricted Antillean endemics.
In general, the Antillean endemics tend to be restricted to forested habitats whereas the continental species occupy open and early successional habitats but exhibit limited penetration of deep forest and montane habitats. Comparisons of habitat distributions of the different groups in the Lesser Antilles and on the continent, represented here by Trinidad, allow us to test certain hypotheses concerning habitat change (Fig. 12). For example, our point count data do not reveal marked differences in the characteristics of species that have colonised the Lesser Antilles versus non-colonising species on Trinidad, seen by comparing groups A and B with non-colonisers. However, once these species arrive on the Lesser Antilles they exhibit ecological release, as seen by contrasting species in groups A and B on Trinidad and in the Lesser Antilles. Subsequent persistence in the Lesser Antilles is not associated with further increase in habitat breadth (compare A and B species with C and D species within the Lesser Antilles). However, recently expanded Antillean endemics have significantly broader habitat distributions than older, restricted endemics (compare C and D species).
Habitat changes associated with the history of birds in the Lesser Antilles are summarised in Table 3. Invaders from the continent exhibit ecological release in habitat breadth upon colonising the islands, which is followed over longer evolutionary periods associated with the evolution of endemic status with a shift into more forested and montane habitats. Secondary expansion of endemic taxa within the Lesser Antilles is associated with a dramatic increase in habitat breadth, perhaps indicative of increased population productivity. Somewhat surprisingly, local density appears to be unrelated to a species’ history and geographic distribution, perhaps owing to strong density-dependent control through territorial defence.
EXTINCTION OF LESSER ANTILLEAN ISLAND POPULATIONS
One issue we haven’t addressed yet is extinction. Gaps in the distributions of many group D species suggest that these taxa have suffered extinction. For example, Adelaide’s warbler is currently confined to the islands of Puerto Rico, Barbuda, and St Lucia, with a genetic distance between the latter two islands of about 2.3% (Lovette et al. 1998). It is plausible that populations of this species occupied intervening islands in the past. The fact that nearly a third of Antillean endemics, the group C species, have undergone expansions during the past half million years suggests that present-day group D species are likely to have undergone similar expansion phases in the past, particularly if they presently occur on more than one island.
If we assume that the present distributions of group C species provide an estimate of the past distributions of group D species, we can then estimate the number of extinctions of island populations among group D species to the present (Fig. 13). Accordingly, it is clear that fewer populations have become extinct on the large, core islands of the central Lesser Antilles than on the smaller islands to the north and south.
Fig. 14 shows for each species the proportion of island populations remaining, out of a total of ten islands considered (weighted by the proportion of group C species on each island), as a function of genetic distance. Thus, it depicts a survival curve for island populations, primarily for group D species, where each species represents an independent cohort of island populations that decreases in number with time. Assuming a constant rate of extinction, the data can be fit by an exponential function, as shown. The estimated extinction rate is 25% for each 1% of nucleotide substitution, or approximately 50% per million years. Accordingly, the average ‘life span’ of a group-D island population on the core islands of the Lesser Antilles is about 2 million years. This estimate is only an overall average and does not take into account heterogeneity in extinction rate among ecological groups of birds or among islands, particularly with respect to island size.
We can use a maximum likelihood approach to estimate the extinction rate of populations on a particular island if we assume that missing group C and group D taxa existed on the island at some time in the past. Fig. 15 shows the ages of extant populations (top row of symbols) and presumably extinct populations (bottom row) on the island of St Vincent. The likelihood of this particular pattern of ages of extant and extinct species is maximised by an exponential extinction rate of 28% per 1% of sequence divergence. The survival curve is indicated by the solid line on the graph, for which the extinction rate is close to the estimate previously derived from the survivorship function for island populations within each species.
Similar estimates for the other major islands in the Lesser Antilles show that extinction rate is inversely related to island area, as one might expect (Fig. 16). Two islands seem somewhat anomalous. One is Montserrat, the smallest of the sample, which shows a low extinction rate and supports an endemic species, the Montserrat oriole. The low apparent extinction rate on Montserrat may result from colonisation, or rescue (Brown & Kodric-Brown 1977), from nearby Guadeloupe. Except for the Montserrat Oriole Icterus oberi, populations on Montserrat are undifferentiated from conspecific populations on Guadeloupe (Lovette et al. 1999a). The second anomalous point is Grenada, which has a high rate of apparent extinction in spite of having a relatively diverse avifauna, which is in large part recently derived from continental South America (group A and B species).
The phenomena related to the taxon cycle seem quite clear. Taxa undergo phases of increasing habitat diversity within islands and increasing geographical distribution within the Lesser Antilles. Expanding endemic taxa are not clearly associated with particular habitats, but occupy a greater variety of habitats than contracting taxa. The dates of origin of expansion phases in the contemporary taxa of the Lesser Antilles are distributed over the past 5 million years, but expansion phases appear not to last more than about a half million years and probably a good deal less. Because expanded and contracted taxa show no particular ecological distinctions, except with respect to habitat breadth, it is unlikely that taxon cycles are driven by climate change. Genetic differentiation of supposedly neutral mitochondrial DNA markers between island populations becomes recognisable within a few hundred thousand years, and perhaps much more quickly. Expansion phases soon lead to periods of population decline and extinction, but secondary expansion also is possible. In spite of the relatively small size of the islands in the Lesser Antilles, some lineages have persisted for very long periods in very dynamic landscapes.
Although older island populations appear to be more vulnerable to anthropogenic extinction, it is unclear whether the rate of natural extinction increases with age, that is, whether populations exhibit ‘senescence’. Maximum likelihood analyses on some islands suggest that models with accelerated extinction rates fit the data better than a simple exponential model with a constant extinction rate (unpublished data). But these results are tentative and further resolution of this issue will await the compilation of genetic distance estimates for the remaining species in our sample.
At present, our working hypothesis is that whether a taxon is currently expanding or contracting depends on the balance between its gene pool and the gene pools of its enemies. Populations exhibit evolutionary inertia occasionally punctuated by the appearance of new genetic variation. It is conceivable, particularly in simplified island ecosystems, that small genetic changes in pathogen virulence or host resistance could profoundly effect population productivity, jolting populations out of equilibrium into phases of expansion or decline towards extinction. Accordingly, it may be relevant that nearly 30% of the Lesser Antillean endemics are in expansion phases (group C), whereas fewer than 10% of the resident land birds of Trinidad have invaded the islands (groups A and B). It may be much more difficult to gain a competitive edge in a diverse, complex community.
Island populations achieve evolutionary independence rather early in the taxon cycle as gene flow between islands decreases relative to selection and drift within islands. Therefore, one would expect the fates of individual island populations to be largely independent, particularly if their fates depend on evolutionary interactions with their enemies.
HOST–PARASITE INTERACTIONS IN LESSER ANTILLEAN BIRDS
We are beginning to see an indication of the evolutionary independence of island populations by examining the occurrence of blood parasites (Apanius et al. 1999). An analysis of the prevalence of Haemoproteus in six species on three islands in the Lesser Antilles revealed a strong species effect, as one might expect, and no island effect (Fig. 17). Most importantly, the significant species-times-island interaction demonstrates that island populations differ in parasite prevalence independently of the characteristics of the species or the island. This is readily apparent in Coereba, Loxigilla, and Vireo.
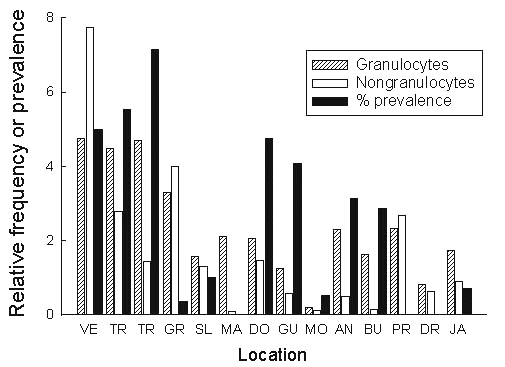
A more detailed picture of inter-island variation in the Bananaquit, which is the most abundant species of bird in the West Indies, shows considerable variation in the prevalence of Haemoproteus. The activity of the immune system as indicated by the frequencies of white blood cells also varies widely among islands (Fig. 18). Paying attention to the prevalence of Haemoproteus, one notices that this tends to be uniformly high in continental sites in Venezuela and Trinidad, but is extremely variable among Antillean islands even where we have found no genetic divergence in mtDNA sequences between islands. Greater Antillean Bananaquits, which are genetically distant from Lesser Antillean populations, are virtually free of Haemoproteus; the same appears to be true of several islands in the Lesser Antilles.
The activity of the immune system shows similarly striking patterns, being uniformly high on the continent and lower and more variable among the islands. The strong immune system activity of birds on Grenada suggests the possibility that this population may be beset by a greater variety of disease organisms brought by the more frequent colonists to that island from the mainland. This may explain the general absence of Group D species from Grenada and the high apparent extinction rate on that island. Following this line of reasoning, one is tempted to suggest that the enigmatic absence of the Bananaquit from Cuba is possibly due to the presence there of an intolerable pathogen.
CONCLUSIONS
This overview of the long-term dynamics of birds in the Lesser Antilles gives a partial picture of the phenomenon of the taxon cycle. The cycle is played out over periods of hundreds of thousands to millions of years. Phases of expansion and contraction are not clearly related to factors in the physical environment, and they more likely reflect evolutionary interactions between birds and their enemies. Thus, taxon cycles are an intrinsic property of biological systems much as predator–prey cycles depend on the dynamics of the interactions between species. The fact that a strong historical signal can be detected in the ecology of very different kinds of species suggests a remarkable homogeneity in progress through the taxon cycle. This homogeneity is consistent with an intrinsic counter-evolutionary nature of the taxon cycle driven by genetic mutation rather than changes in the physical environment.
Island populations within the Lesser Antilles for the most part become evolutionarily independent soon after their colonisation. Populations persist for shorter periods on small islands than on large islands, but the reasons for this are not clear. If the colonisation event can be compared to birth, then populations do change with age, losing the vigour of the expansion phase and presumably becoming increasingly vulnerable to extinction unless rescued by the chance appearance of new genetic variation. This scenario is still partly imagined, although its basic outline is becoming clear. More importantly, genetic analysis has given us a handle on the time dimension of the taxon cycle, and will likely help us to understand the evolutionary mechanisms of the taxon cycle in the future.
ACKNOWLEDGEMENTS
Fieldwork to collect blood and tissue samples has been generously supported by the National Geographic Society (grants 4436-90, 4951-93, 5377-94). The Smithsonian Institution and the National Science Foundation (DEB-9596272) have funded laboratory work. Gilles Seutin and Irby Lovette have been invaluable collaborators in many phases of this work, as have a number of field and laboratory assistants, particularly Jeff Hunt. Dolph Schluter and Trevor Price provided insightful comments on the manuscript. None of this work would have been possible without the co-operation and interest of numerous government officials and conservation biologists throughout the West Indies.
REFERENCES
Abbott, I. 1980. Theories dealing with the ecology of landbirds on islands. Advances in Ecological Research 1: 329–371.
Apanius, V., Yorinks, N., Bermingham, E. & Ricklefs, R.E. 1999. Island and taxon effects in parasitism and resistance of Lesser Antillean birds. Ecology, in press.
Bermingham, E., Rohwer, S., Freeman, S. & Wood, C. 1992. Vicariance biogeography in the Pleistocene and speciation in North American wood warblers: a test of Mengel’s model. Proceedings of the National Academy of Sciences USA 89: 6624–6628.
Bermingham, E., Seutin, G. & Ricklefs, R.E. 1996. Regional approaches to conservation biology: RFLPs, DNA sequence, and Caribbean birds. In: Smith, T.B. & Wayne, R.K. (eds) Molecular Genetic Approaches in Conservation. New York; Oxford University Press: 104–124.
Bond, J. 1956. Checklist of Birds of the West Indies, Philadelphia: Academy of Natural Sciences.
Brown, J.H. 1995. Macroecology, Chicago: University of Chicago Press.
Brown, J.H. & Kodric-Brown, A. 1977. Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58: 445–449.
Brown, J.H. & Maurer, B.A. 1989. Macroecology: the division of food and space among species on continents. Science 243: 1145–1150.
Bush, G.L. 1975. Modes of animal speciation. Annual Review of Ecology and Systematics 6: 339–364.
Bush, M.B., Weimann, M., Piperno, D.R., Liu, K.-B. & Colinvaux, P.A. 1990. Pleistocene temperature change and vegetation depression in Ecuadorian Amazonia. Quaternary Research 34: 330–345.
Cox, G.W. & Ricklefs, R.E. 1977. Species diversity, ecological release, and community structuring in Caribbean land bird faunas. Oikos 29: 60–66.
Donnelly, T.W. 1989. Geologic history of the Caribbean and Central America. In: Bally, A.W. & Palmer, A.R. (eds) The Geology of North America – an Overview. Boulder, Colorado; The Geological Society of America: 299–321.
Eldredge, N. & Gould, S.J. 1972. Punctuated equilibria: an alternative to phyletic gradualism. In: Schopf, T.J.M. (ed.) Models in Paleobiology. San Francisco; Freeman, Cooper & Co: 82–115.
Finch, C.E. 1990. Longevity, Senescence, and the Genome, Chicago: University of Chicago Press.
Fleischer, R.C., McIntosh, C.E. & Tarr, C.E. 1998. Evolution on a volcanic conveyor belt: using phylogeographic reconstructions and K-Ar-based ages of the Hawaiian Islands to estimate molecular evolutionary rates. Molecular Ecology 7: 533–545.
Gould, S.J. & Eldredge, N. 1977. Punctuated equilibria: the tempo and mode of evolution reconsidered. Paleobiology 3: 115–151.
Haldane, J.B.S. 1957. The cost of natural selection. Journal of Genetics 55: 511–524.
Hamilton, W.D. 1991. The seething genetics of health and the evolution of sex. In: Osawa, S. & Honjo, T. (eds) Evolution of Life: Fossils, Molecules, and Culture. Berlin; Springer-Verlag: 229–252.
Hartl, D.L. & Clark, A.G. 1997. Principles of Population Genetics, 3rd ed. Sunderland, Massachusetts: Sinauer Associates.
Hooghiemstra, H. 1989. Quaternary and upper-Pliocene glaciations and forest development in the tropical Andes: evidence from a long-high-resolution pollen record from the sedimentary basin of Bogota, Colombia. Palaeogeography, Palaeoclimatology, Palaeoecology 72: 11–26.
Klein, N.K. & Brown, W.M. 1994. Intraspecific molecular phylogeny in the yellow warbler (Dendroica petechia), and implications for avian biogeography in the West Indies. Evolution 48: 1914–1932.
Klicka, J. & Zink, R.M. 1997. The importance of recent ice ages in speciation: a failed paradigm. Science 277: 1666–1669.
Lawton, J.H., Nee, S., Letcher, A.J. & Harvey, P.H. 1994. Animal distributions: patterns and processes. In: Edwards, P.J., May, R.M. & Webb, N.R. (eds) Large-Scale Ecology and Conservation Biology. Oxford; Blackwell: 41–58.
Liebherr, J.K. & Hajek, A.E. 1990. A cladistic test of the taxon cycle and taxon pulse hypotheses. Cladistics 6: 39–59.
Lovette, I.J., Bermingham, E. & Ricklefs, R.E. 1999. Mitochondrial DNA phylogeography and the conservation of endangered Lesser Antillean Icterus orioles. Conservation Biology, in press.
Lovette, I.J., Bermingham, E., Seutin, G. & Ricklefs, R.E. 1998. Evolutionary differentiation in three endemic West Indian warblers. Auk 115: 890–903.
Lovette, I.J., Bermingham, E., Seutin, G. & Ricklefs, R.E. 1999. The origins of an island fauna: a genetic assessment of sources and temporal patterns in the avian colonisation of Barbados. Biological Invasions, in press.
Mayr, E. 1963. Animal Species and Evolution, Cambridge, MA: Harvard University Press.
Montserrat Volcano Observatory Team. 1997. The ongoing eruption in Montserrat. Science 276: 371–372.
Pielou, E.C. 1979. Biogeography, New York: Wiley.
Pregill, G.K. & Olson, S.L. 1981. Zoogeography of West Indian vertebrates in relation to Pleistocene climatic cycles. Annual Review of Ecology and Systematics 12: 75–98.
Ricklefs, R.E. & Bermingham, E. 1997. Molecular phylogenetics and conservation of Caribbean birds. El Pitirre 10: 85–92.
Ricklefs, R.E. & Cox, G.C. 1972. Taxon cycles in the West Indian avifauna. American Naturalist 106: 195–219.
Ricklefs, R.E. & Cox, G.W. 1978. Stage of taxon cycle, habitat distribution, and population density in the avifauna of the West Indies. American Naturalist 112: 875–895.
Rose, M.R. 1991. Evolutionary Biology of Aging, New York: Oxford University Press.
Seutin, G., Brawn, J., Ricklefs, R.E. & Bermingham, E. 1993. Genetic divergence among populations of a tropical passerine, the streaked saltator (Saltator albicollis). Auk 110: 117–126.
Seutin, G., Klein, N.K., Ricklefs, R.E. & Bermingham, E. 1994. Historical biogeography of the Bananaquit (Coereba flaveola) in the Caribbean region: a mitochondrial DNA assessment. Evolution 48: 1041–1061.
Shields, G.F. & Wilson, A.C. 1987. Calibration of mitochondrial DNA evolution in geese. Journal of Molecular Evolution 24: 212–217.
Stanley, S.M. 1979. Macroevolution. Pattern and Process, San Francisco: W.H. Freeman.
Tarr, C.L. & Fleischer, R.C. 1993. Mitochondrial-DNA variation and evolutionary relationships in the amahiki complex. Auk 110: 825–831.
Tarr, C.L. & Fleischer, R.C. 1995. Evolutionary relationships of the Hawaiian honeycreepers (Aves, Drepanidinae). In: Wagner, W.L. & Funk, V.A. (eds) Hawaiian Biogeography. Washington, D.C.; Smithsonian Institution Press: 147–159.
Van Valen, L.M. 1973. A new evolutionary law. Evolutionary Theory 1: 1–30.
Williamson, M. 1981. Island Populations, Oxford: Oxford University Press.
Wilson, E.O. 1961. The nature of the taxon cycle in the Melanesian ant fauna. American Naturalist 95: 169–193.
Wunderle, J.M. 1985. An ecological comparison of the avifaunas of Grenada and Tobago, West Indies. Wilson Bulletin 97: 356–365.
Table 1. Criteria for assigning species to stages of the taxon cycle according to Ricklefs & Cox (1972).

Table 2. Criteria for assigning species of Lesser Antillean birds to distributional groups based on continental (or Greater Antillean) versus endemic origin, and relative geographic distribution with the Lesser Antilles.
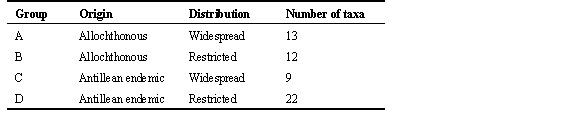
Table 3. Statistical comparisons designed to test the hypotheses that (1) island invaders have different habitat distributions on the mainland than non-invaders, i.e. Trinidadian species that have not colonised the Lesser Antilles, (2) recently expanded Antillean endemics have different habitat distributions than contracting species, (3) that invading species from the mainland exhibit ecological release on colonising the Lesser Antilles, and (4) that ecological distributions change with time, independently of phases of expansion and contraction, after colonization of the Lesser Antilles. Comparisons with less than (<) and greater than (>) symbols (in boldface) indicate significant differences assessed by ANOVA.

Fig. 1. Representative distributions of non-raptorial land birds across the major islands in the Lesser Antilles, on Tobago, and on the continental land mass of Trinidad. Letters next to island populations of the Lesser Antillean Bullfinch and the House Wren indicate named subspecies. The populations of Adelaide’s Warbler on St Lucia and Barbuda also are strongly differentiated. The Gray Kingbird is distributed throughout the West Indies; Adelaide’s Warbler also occurs on Puerto Rico.
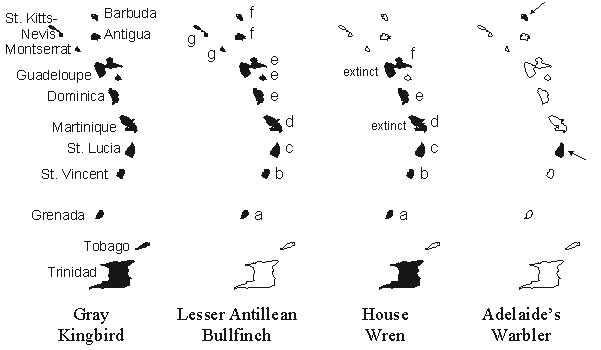
Fig. 2. Diagram of the progression of species from stage 1 to stage 3, and back to stage 1, of the taxon cycle. Because no species in the Lesser Antilles with a fragmented distribution is taxonomically undifferentiated, we assume that the sequence from stage 1 to stage 3 involves differentiation (stage 2) followed by extinction of island populations. If movement between islands is frequent, taxa may remain in stage 1 without progressing through the cycle. Further extinction of island populations may convert stage 3 to stage 4 and eventually lead to global extinction if no re-expansion phase occurs.
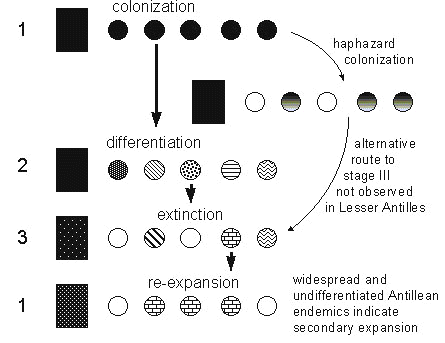
Fig. 3. Percentage of island populations that are either extinct or threatened by extinction for taxa in each stage of the taxon cycle in the West Indies, Galapagos Archipelago, and Hawaiian Islands. Data for the West Indies are from Ricklefs & Cox (1972); data for Galapagos and Hawaii were compiled from the literature by R.E. Ricklefs (unpubl. data). The status of many of the Hawaiian species is uncertain because of human-caused extinctions prior to scientific collecting in the islands. However, the pattern with respect to taxon cycle stage is robust to different interpretations of taxonomy and distribution.
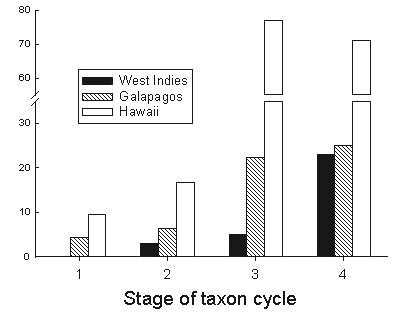
Fig. 4. Phylogeography of the Bananaquit in the West Indies. The root of the phylogeny of Bananaquit lineages is in the Greater Antilles and Bahamas. Bananaquits most likely colonised the Lesser Antilles from Puerto Rico. Within the Lesser Antilles, the southern islands of St Vincent and Grenada are genetically distinct. There appears to be a recent expansion from Dominica north to the Virgin Islands, involving a founder event between Dominica and Guadeloupe. Bananaquits on Barbados, the easternmost of the Lesser Antilles, are closely related to those on St Lucia, rather than St Vincent. Points A and B indicate postulated colonisation of the Lesser Antilles from the Greater Antilles and the beginning of the expansion phase that produced the contemporary island populations in the Lesser Antilles (Fig. 5). After Seutin and Bermingham, in review.
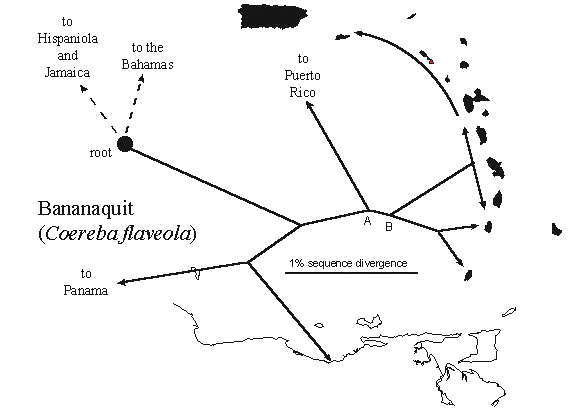
Fig. 5. Diagram of the phylogenetic nodes (branch points) used to characterise the timing of the beginning of the expansion phase of a taxon that resulted in its present-day island populations in the Lesser Antilles and the original colonisation of the taxon from the continent or Greater Antilles. These points are indicated by B and A in Fig. 4.
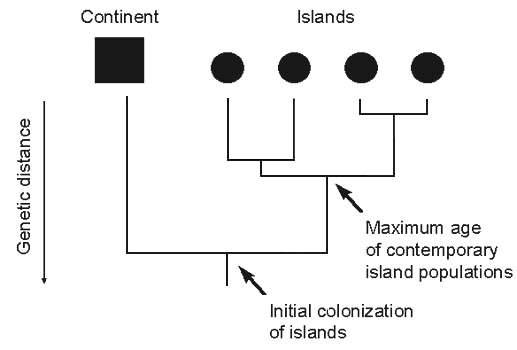
Fig. 6. Genetic distances representing the initiation of the contemporary expansion phases of species grouped according to stage of taxon cycle. In the case of stage 4 species, the distance is to the closest sister taxon. Data are presented as means and standard deviations to indicate the range of variation. Means differ significantly among stages (F3,21 = 8.35, P = 0.0008, R2 = 0.54).
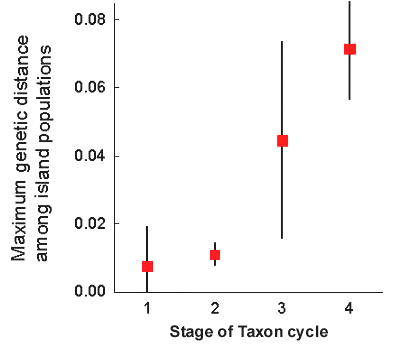
Fig. 7. Representative patterns of habitat occupancy of three island populations of Lesser Antillean birds determined by point counts (presence or absence during 20 minute stops at each of 10 stations in each of 7 habitat types). Habitat diversity is calculated as 1/ ĺpi 2, where pi is the proportion of counts in the ith habitat. Average position is the weighted average of the habitat score (1-7). Density is the average number of counts within each occupied habitat (1-10).
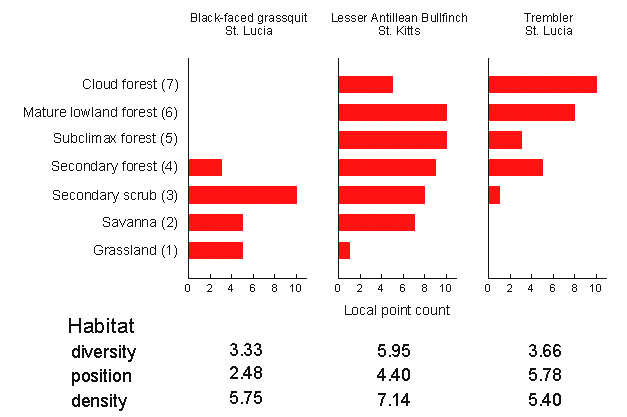
Fig. 8. Average values of habitat diversity, habitat position, and local density as a function of stage of taxon cycle. Data are presented as means and standard errors. Statistical tests were determined in nested ANOVA based on the F-ratio of stage/species(stage): diversity (F3, 49 = 5.2, P = 0.0035); position (F3, 36 = 4.6, P = 0.0065); density (F3, 46 = 2.1, P = 0.12). t-tests of least-squares means identified the following significant differences: diversity (stages 1 and 2 vs. 3 and 4); position (1 vs. 2 vs. 3,4); density (1,2 vs. 3,4).
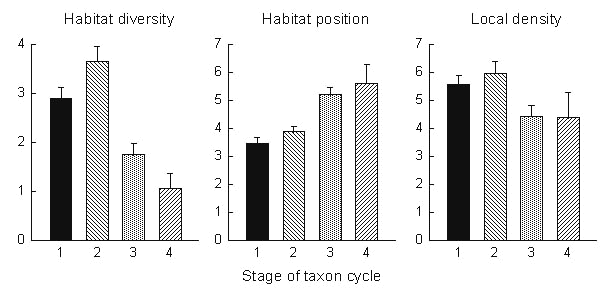
Fig. 9. Distributions of species in each of the distribution groups recognised in the Lesser Antilles: (A) authochthonous and widespread, the Gray Kingbird Tyrannus dominicensis; (B) authochthonous and restricted, the Bare-eyed Thrush Turdus nudigenis;(C) Lesser Antillean and widespread, the Lesser Antillean Bullfinch Loxigilla noctis; (D) Antillean and restricted, the Forest Thrush Cichlherminia lherminieri.
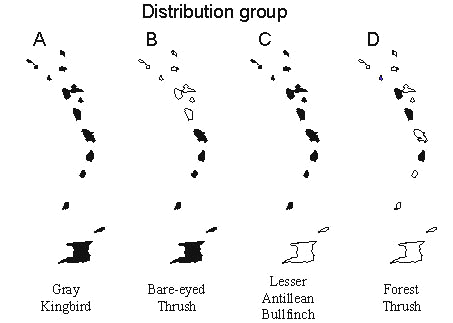
Fig. 10. Number of species in each distribution group on each island in the Lesser Antilles and adjacent regions. TR = Trinidad, TO = Tobago, GR = Grenada, SV = St Vincent, SL = St Lucia, MA = Martinique, DO = Dominica, GU = Guadeloupe, MO = Montserrat, AN = Antigua, BU = Barbuda, SK = St Kitts, PR = Puerto Rico.
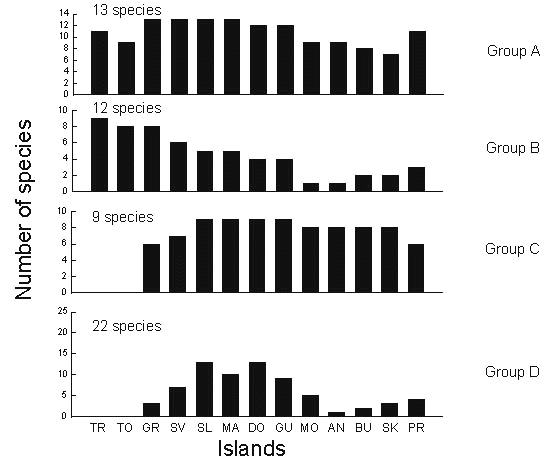
Fig. 11. Genetic distances representing the initiation of the contemporary phase of expansion within the Lesser Antilles according to distribution group. For group C species, the relative timing of the contemporary expansion phase and the initial colonisation are indicated. Data are presented as means and standard deviations to indicate the range of variation. Differences in relative ages of most recent expansions are statistically significant (F3, 21 = 12.9, P = 0.0001, R2 = 0.65).
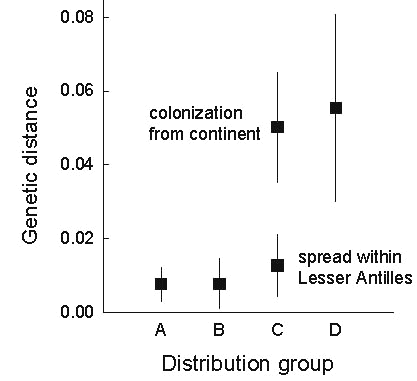
Fig. 12. Mean (±SE) habitat diversity of birds within the continental island of Trinidad and on the Lesser Antillean islands of Grenada, St Lucia, Dominica, and St Kitts with respect to distribution group. A and B species differ significantly between Trinidad and the Lesser Antilles (F1, 42 = 7.22, P = 0.01). Within Trinidad, group A and B species do not differ from non-invaders, i.e., Trinidadian species that have not colonised the Lesser Antilles. Within the Lesser Antilles, A,B do not differ from C,D, but C and D differ significantly (Duncan’s Multiple Range Test).
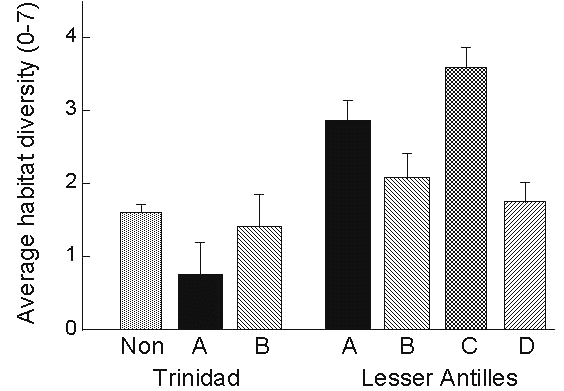
Fig. 13. Numbers of group C and group D species on islands in the Lesser Antilles. The open bars represent the distributions of the 22 group D species prior to extinction if they were initially distributed the way group C species are at present. The proportion of group D island populations presumed extinct on each island is the open portion of each bar divided by its total height. Island acronyms as in Fig. 10.
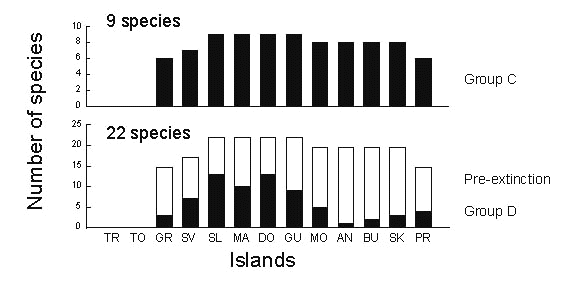
Fig. 14. Proportion of island populations extant as a function of maximum genetic divergence of island populations of species of Lesser Antillean bird. Each symbol represents a single species. Closed symbols: group A and B species; open symbols: group C and D species. The exponential curve fitted to the data (Y = e–mX) has an extinction rate (m) of 0.25 (±0.02 SE) per percentage of genetic distance (X), or about 50% per million years.
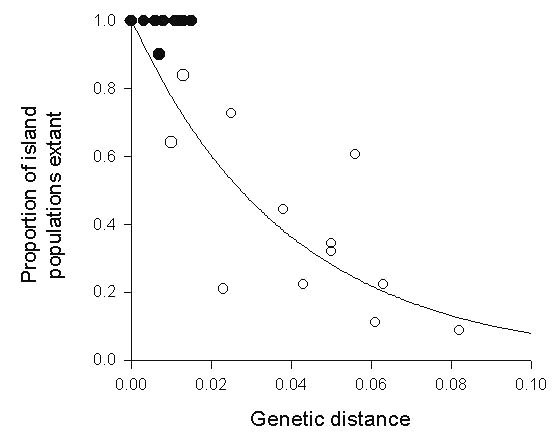
Fig. 15. The relative ages (maximum genetic distance within the Lesser Antilles) of extant (top row) and putatively extinct (bottom row) group C (d < 2%) and group D (d > 2%) species on the island of St Vincent. The extant species with a genetic distance of 8.2% is the endemic Whistling Warbler Catheropeza bishopi. The exponential population survival curve (extinction rate = 0.28 per percent of genetic distance) is a maximum likelihood estimate. The likelihood that a population is extant at a particular genetic distance is L = e–md , and the likelihood that a population is extinct is L = 1 – e–md. The total likelihood is ĺ logeL for all species.
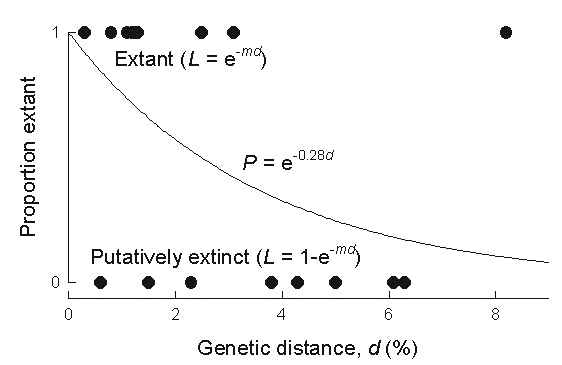
Fig. 16. Maximum likelihood estimates of extinction rates of non-raptorial land birds on several islands in the Lesser Antilles plotted as a function of island size (log scale). The regression of the logarithm of extinction rate on the logarithm of area has a slope of –0.61 (±0.23 SE) (F1,8 = 7.3, P = 0.027, R2 = 0.48).
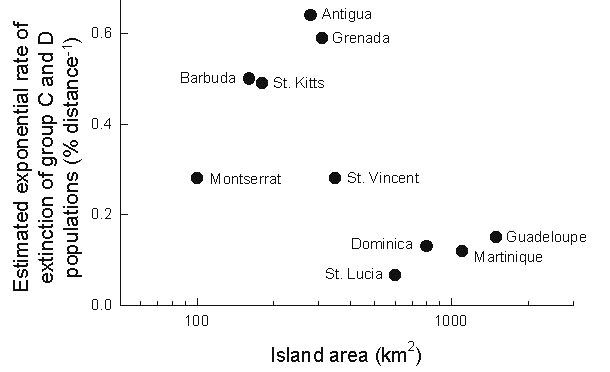
Fig. 17. Prevalence of Haemoproteus in populations of six species on three islands in the Lesser Antilles. A categorical model of prevalence indicated a significant effect of host species (c 25 = 40.4, P < 0.0001), but not of island (c 22 = 2.3, P = 0.31), and a strong species × island interaction (c 210 = 28.1, P = 0.002). From Apanius et al. 1999.
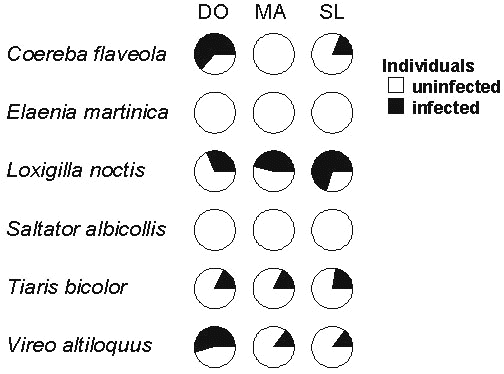
Fig. 18. Prevalence of Haemoproteus and relative numbers of white blood cells in populations of the Bananaquit on islands in the Lesser Antilles and adjacent areas. VE = Venezuela (two localities), JA = Jamaica, other acronyms as in Fig. 10. Antibody production is restricted to certain classes of nongranulocytes; phygocytosis is found in classes of both granulated and nongranulated cells. Data compiled from blood smears by Ascanio Castillo.