Plenary08: Cuckoos and cowbirds versus hosts: Co-evolutionary lag and equilibrium
Nicholas B. Davies
Department of Zoology, University of Cambridge, Downing Street, Cambridge, CB2 3EJ, UK, e-mail n.b.davies@zoo.cam.ac.uk
Davies, N.B. 1999. Cuckoos and cowbirds versus hosts: Co-evolutionary lag and equilibrium. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban. Ostrich 70 (1): 71–79.
Experiments show co-evolution between the Common Cuckoo Cuculus canorus and its hosts. Both egg mimicry and laying behaviour of cuckoos have evolved in relation to host defences. In turn, host egg rejection and aggression to cuckoos have evolved in response to parasitism. Selective egg replacement by cuckoos may also lead to egg mimicry but current evidence for this is weak. Hosts incur costs of rejection, so below a critical parasitism frequency they do better to accept. This is reflected in phenotypic flexibility in host defences in relation to small-scale geographical variation and temporal changes in parasitism rate. The puzzle is why so many hosts accept non-mimetic eggs. There are more acceptors among cowbird hosts in North and South America than among cuckoo hosts in Europe or southern Africa, and cowbird hosts show less intermediate rejection frequencies. One hypothesis is that acceptor hosts would do better to reject and accept because they are at the start of the co-evolutionary cycle. In support of this: host–parasite systems show dynamic changes, calculations suggest that many hosts would indeed do better to reject, and old cowbird hosts are stronger rejectors than are new hosts. An alternative hypothesis is that host acceptance can be an equilibrium. In support of this: rejection costs for some hosts are sufficiently high for acceptance to be best, in Australia a long breeding season reduces parasitism costs and may explain acceptance there, and Mafia cuckoos may enforce acceptance. Variation in host acceptance is likely to reflect a mixture of systems at equilibrium and those showing evolutionary lag. Host responses to parasite chicks are discussed, particularly how the parasite chicks manipulate hosts through begging signals.
INTRODUCTION
The interactions between brood parasites and their hosts provide a puzzling mixture of exquisite adaptations and strange lack of adaptations. In some cases the hosts reject eggs unlike their own and the parasite has evolved near-perfect egg mimicry. In other cases, however, the parasite’s egg looks very different from the host’s eggs, yet the hosts accept it. This diversity is well illustrated by South African cuckoos and their hosts (Rowan 1983). For example, Black Cuckoos Cuculus clamosus lay eggs which mimic those of their hosts, Laniarius shrikes, and these reject non-mimetic eggs (Noble 1995). Likewise, the various gentes (host-specific strains) of the Diederik Cuckoo Chrysococcyx caprius lay mimetic eggs for their respective weaver, sparrow and bishop hosts (Ploceidae), and again these hosts reject eggs unlike their own (Victoria 1972; Noble 1995; Lawes & Kirkman 1996; Lindholm 1997). However, there are equally striking examples of cuckoos laying non-mimetic eggs for their major hosts and of the hosts apparently tolerating them. For example, the plain white egg of the Jacobin Cuckoo Oxylophus jacobinus contrasts markedly with the spotted eggs of its bulbul Pycnonotus spp. hosts, and the uniform brown egg of the Red-chested Cuckoo Cuculus solitarius differs from the paler and spotted eggs of its main host the Cape Robin Cossypha caffra and from most of its other thrush hosts (Payne & Payne 1967).
There is similar diversity of adaptation at the chick stage. Sometimes the parasite chicks show remarkable mimicry of the host young: Vidua finches parasitize other estrildid finches and the parasite young mimic the mouth patterns and colours of the host nestlings with which they are raised in a mixed brood (Nicolai 1964). In other cases, however, the parasitic chick is strikingly different from the host young. The young Common Cuckoo Cuculus canorus has a much brighter, redder mouth than that of its host’s nestlings, yet even those hosts which reject non-mimetic eggs never reject the cuckoo chick, and they continue to feed it even as it grows to six times their own body weight.
These observations raise a fascinating general question: when we see an animal apparently doing something crazy, like feeding a monstrous-sized parasitic chick or accepting a parasitic egg so different from its own, is this because of a time lag in adaptation during a co-evolutionary cycle between parasite and host, or could these be stable outcomes, which reflect constraints on the mechanisms by which individuals guide their behaviour? The aim of this paper is to discuss recent studies of brood parasitism in the light of these alternatives. I begin by reviewing work on co-evolution between the Common Cuckoo and its hosts, a well-studied system which reveals the costs and benefits to hosts of counter adaptations to parasitism.
CO-EVOLUTION BETWEEN THE COMMON CUCKOO AND ITS HOSTS
Cuculus canorus and its hosts
Individual female Common Cuckoos tend to favour one particular host species and lay distinctive eggs, which match to varying degrees the eggs of their respective hosts (Chance 1940; Baker 1942; Wyllie 1981; Gärtner 1982; Brooke & Davies 1988). There are at least fifteen different cuckoo egg-morphs in Europe (Moksnes & Røskaft 1995), with the major hosts being small passerines which nest in low vegetation (warblers, chats, shrikes, finches) or on the ground (pipits, wagtails, buntings). Thus, Cuculus canorus is apparently divided into genetically distinct strains or ‘gentes’ (singular ‘gens’). We are still ignorant about how these gentes are maintained. It is not known whether they are cryptic species or female-specific host races, though the latter possibility is suggested by Hiroshi Nakamura’s data in Japan (pers. comm.) which shows that individual males mate with females specialising on several hosts. This implies that only females influence their daughter’s egg type, perhaps because the w (sex) chromosome codes for egg characteristics. However, nothing is known of the genetics of egg mimicry. If only female lines of cuckoo are distinct, with males interbreeding with the different gentes to maintain the one species, then there should be differences between gentes in mitochondrial DNA (transmitted through females only) but not in nuclear DNA (which segregates through both sexes). However no genetic differences have been found (Gibbs et al. 1996). Nor do we know how females come to choose the right host species, namely the one for whom their egg-type is a good match. It has long been supposed that this comes about because young female cuckoos imprint on the host that rears them, but so far there is no field evidence that daughter cuckoos select the same host as their mothers (though ringing recoveries suggest some natal philopatry; Seel 1977), and the one experimental test on captive cuckoos failed to reveal host imprinting (Brooke & Davies 1991).
A cuckoo lays on average eight eggs per season (Wyllie 1981). She parasitises the host nest in the late afternoon during the host’s laying period, first removing one host egg and then laying a single egg in its place, her stay at the nest being less than ten seconds (Chance 1940). The cuckoo egg needs a day’s less incubation than the host eggs, so it hatches first. Then, just a few hours old, and still naked and blind, the cuckoo chick ejects the host eggs (and any chicks) from the nest, and so becomes the sole occupant. The host parents then slave away to feed it for about 20 days in the nest and for a further two weeks after fledging (Wyllie 1981).
Evidence for co-evolution
In theory, natural selection should favour host defences, which in turn should promote the evolution of improved trickery by the cuckoo, leading to a co-evolutionary arms race in which each party evolves in response to the other (Fig. 1). Recent experiments provide good evidence for co-evolution.
Two sources of evidence show that the Common Cuckoo has evolved in response to selection from hosts (top arrow in Fig. 1). First, experiments in which we parasitised Reed Warbler Acrocephalus scirpaceus nests with model cuckoo eggs show that the Common Cuckoo’s egg-laying tactics are designed to circumvent host defences (Davies & Brooke 1988). These hosts are more likely to reject non-mimetic eggs than mimetic eggs, and will even reject all mimetic eggs if they are deposited in the nest before the warblers have started their clutch. Mimetic eggs are also more likely to be rejected if they are put in the nest at dawn (when the warblers themselves lay) rather than in the afternoon (when the hosts are more likely to be absent from the nest). Increased rejection is also stimulated by the sight of a stuffed cuckoo on the nest (see also Moksnes & Røskaft 1989; Moksnes et al. 1993). These host defences make good sense of the Common Cuckoo’s laying tactics, namely a mimetic egg, laid in the afternoon, during the host laying period, with extraordinarily fast laying to reduce the chance that it alerts the host.
Second, the degree of egg mimicry shown by the various Common Cuckoo gentes corresponds with the degree of discrimination from their respective hosts (Brooke & Davies 1988; Davies & Brooke 1989a). In Europe, most hosts reject non-mimetic eggs and their Common Cuckoo gens has evolved a reasonably well-matching egg (see Higuchi 1989 for similar results in Japan). The exception is the Dunnock Prunella modularis, which shows no rejection, and in this case the Common Cuckoo gens (despite laying a distinctive egg) shows no colour mimicry (Dunnock eggs are immaculate turquoise blue; the Common Cuckoo egg is pale brown with dense spotting). Thus both Common Cuckoo eggs and Common Cuckoo laying behaviour have evolved in relation to selection from hosts.
Two sources of evidence show that hosts, in turn, have evolved in response to selection from cuckoos (bottom arrow in Fig. 1). First, passerine birds which are unsuitable as hosts, either because they nest in holes (inaccessible to a laying cuckoo) or feed their chicks on seeds (unsuitable for a young cuckoo), show little, if any, rejection of odd eggs from their nest (Davies & Brooke 1989a; Moksnes et al. 1991). Noble (1995) also found that unsuitable hosts in Namibia showed little egg rejection. The fact that species untainted by evolutionary interactions with cuckoos show acceptance, suggests that the rejection behaviour of suitable hosts has evolved specifically in response to cuckoo parasitism. Second, unsuitable hosts also show little aggression to Common Cuckoo mounts, while suitable hosts are not only more aggressive (Moksnes et al. 1991), they also show specific defences suited to the threats posed by Common Cuckoos (Duckworth 1991). For example, adult Reed Warblers attack Common Cuckoos near their nests, but mob hawks from a safe distance and they cease to attack Common Cuckoos once their young fledge. Common Cuckoos are a threat at the egg stage, both as predators and parasites, and they will kill nestlings too, but are no threat to fledglings.
Other host defences which may reflect evolved defences against cuckoos include nest architecture (e.g. a long entrance tube, as in some weaver nests, which may hinder cuckoo entry; Freeman 1988) and both reduced intra-clutch variation and increased inter-clutch variation in host egg colour and speckling (which makes it easier for hosts to discriminate against a foreign egg; Øien et al. 1995; Soler & Møller 1996). However, it is not yet clear to what extent these reflect specific defences against cuckoos as opposed to defences against intra-specific brood parasitism.
There are two further points to make concerning the evolution of egg mimicry. First, ‘perfect mimicry’ may not always be the expected outcome of a cuckoo-host arms race. In experiments with Herring Gulls Larus argentatus Baerends & Drent (1982) found that they could make a model egg which was more attractive to the gulls than one of their own eggs. A ‘supernormal’ egg was one slightly larger and more finely speckled than the gulls’ own eggs. It is intriguing to note that cuckoo eggs tend to be both larger and more finely speckled than host eggs.
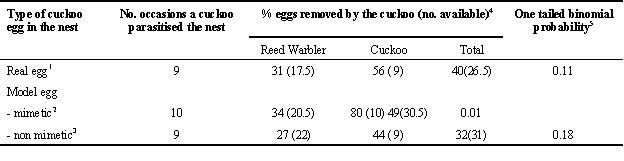
Second, host discrimination may not be the only selective pressure on cuckoo eggs. Cuckoos of the genus Cuculus and Chrysococcyx remove an egg from the host nest before laying their own (Wyllie 1981; Rowan 1983; Lindholm 1997). Our experiments show that this is not selected for by hosts noting an extra egg in their nest (Davies & Brooke 1988, 1989a). Instead, it may reflect competition among cuckoos themselves. Sometimes, two females lay in the same host nest. It would clearly pay the second cuckoo to remove the first cuckoo’s egg, because there is room for only one cuckoo in the nest and the first cuckoo to hatch will eject all the other eggs. Host egg mimicry may evolve, therefore, because it reduces the chance that second cuckoos will be able to discriminate, and remove, a cuckoo egg from the clutch (Davies & Brooke 1988). Table 1 updates our previous analysis to test this hypothesis. It summarises what happens when a cuckoo parasitises a nest containing either a real cuckoo egg or a model cuckoo egg. There was no significant tendency for Common Cuckoos to selectively remove a real Common Cuckoo egg. Curiously, there was a significant tendency to selectively remove a mimetic model egg but not a non-mimetic model, the reverse of what we would predict. Clearly, a larger sample is needed. However, even if selective egg replacement by Common Cuckoos occurs, it is likely to be a much weaker selective force for egg mimicry by Cuculus canorus than host discrimination. For example 14% of parasitised Reed Warbler nests were visited by a second cuckoo on our study site (Davies & Brooke 1988). Even if the cuckoo always removed the first cuckoo’s egg, this rejection rate would be well below that arising from host rejection of non-mimetic eggs (69% nests).
In Australia, however, the selective pressures may be different. In marked contrast to European, Japanese and African studies, Brooker & Brooker (1989) found no evidence for host rejection of non-mimetic eggs. Nevertheless, the Horsfield’s Bronze-Cuckoo Chrysococcyx basalis lays highly mimetic eggs while the Shining-Bronze Cuckoo C. lucidus lays non-mimetic, dark eggs which are difficult to see in the dark interior of the domed nests of their hosts. The Brookers (1990) suggest that in these cases, cuckoo egg replacement may be the only selective pressure on cuckoo egg appearance, selecting either for host egg mimicry or crypsis in the host nest, in both cases to reduce the chances of removal by other cuckoos. Evidence is now needed that Australian cuckoos are selective in their removal of eggs. The Brookers (1990, pers. comm.) favour selective cuckoo egg removal as the major pressure for the evolution of host egg mimicry in other cuckoos too, but the evidence in the previous paragraph argues against this. Furthermore, some excellent examples of host egg mimicry occur in Jacobin Cuckoos Oxylophus jacobinus in India (Gaston 1976) and Great Spotted Cuckoos Clamator glandarius in Europe (Soler et al. 1997) and in neither case do the cuckoos remove an egg from the host nest. Nevertheless, the Australian results highlight the importance of testing host responses to investigate co-evolution, and suggest that egg mimicry in different host–parasite systems may involve different balances of selective forces.
Costs of egg rejection and flexible host defences
Once the cuckoo has evolved egg mimicry, the host has the problem of recognising whether there is a cuckoo egg in its clutch. Experiments with model eggs have shown that hosts learn what their own eggs look like and then reject eggs which differ from this learned set (Rothstein 1974, 1975a, 1978; Lotem et al. 1992, 1995). Among the features used to discriminate foreign eggs are differences in background colour, spotting and size (Rothstein 1982a). However, hosts sometimes make recognition errors when faced with mimetic eggs and reject one or more of their own eggs rather than the foreign egg (Davies & Brooke 1988; Marchetti 1992).
We calculated the costs and benefits of egg rejection for Reed Warbler hosts of Cuculus canorus (Davies et al. 1996). An unparasitised nest contains four host eggs (the average clutch size). A parasitised nest contains three host eggs and one cuckoo egg (the cuckoo replaces one host egg with its own). An ‘acceptor’ host gains the reproductive success from four eggs if it is not parasitised, and a payoff of zero if it is parasitised (because the cuckoo chick ejects the host eggs). A ‘rejector’ host faced with a mimetic cuckoo egg makes recognition errors in 30% of rejections. In these cases its payoff is zero, because the cuckoo remains in the nest. In the other 70% cases it correctly ejects the cuckoo egg, along with an average of 0.5 of its own eggs, which it cracks while ejecting the cuckoo egg (an ‘ejection’ cost). So it is left with 2.5 of its own eggs in the nest. Its average payoff is therefore (0.7 × 2.5) + (0.3 × 0) = 1.75. In the absence of parasitism, we assume that a ‘rejector’ makes recognition errors in the same 30% cases and observations show that 1.2 own eggs are rejected in these cases, so the payoff is (0.7 × 4) + (0.3 × 2.8) = 3.6. Clearly then, given these recognition costs it is best for Reed Warblers to reject if they are parasitised (1.75 > 0) and accept if they are not parasitised (4 > 3.6). The critical parasitism frequency above which it pays to reject mimetic eggs is 19%. Below this, hosts do better to accept.
These calculations help to explain why Reed Warblers vary their rejection behaviour of mimetic model Common Cuckoo eggs (Davies & Brooke 1988). If the warblers do not see a Common Cuckoo at their nest, then natural selection should favour the best response for the average parasitism rate for the population. On our fenland study sites, parasitism was 22% in 1985 and 9% in 1986, and over the whole of Britain it is 6% (Brooke & Davies 1987). These rates tend to fall below the predicted threshold for rejection, which fits with the fact that most mimetic model eggs (97%) and real Common Cuckoo eggs (81%) are accepted. When we placed a stuffed Common Cuckoo on the nest, the Warblers increased their rejection to 56% cases. If the sight of a Common Cuckoo on the nest is a certain predictor of parasitism, our calculations suggest that the warblers should have always rejected in this experiment. However, Common Cuckoos sometimes visit nests to inspect them rather than to lay (Chance 1940). Until we know more about how the hosts themselves assess parasitism rates, it is difficult to test the model quantitatively. Nevertheless, the main conclusion is that costs of rejection mean that it will pay hosts to be flexible in their responses depending on their chances of parasitism.
Recent studies provide further support for the idea of flexible host defences. First, unparasitised populations of Reed Warblers show less rejection of model Common Cuckoo eggs than do nearby parasitised populations (Brooke et al. 1998; Lindholm & Thomas 1999). This raises the possibility that lower rejection in more isolated unparasitised populations, for example wagtails Motacilla alba and pipits Anthus pratensis in Iceland (Davies & Brooke 1989a), may also reflect phenotypic plasticity rather than genetic differences, which we first assumed (for a similar result with cowbird hosts, see Briskie et al. 1992). Second, on our main fenland study site, Wicken Fen, there has been a decline in Common Cuckoos during the last decade, so that parasitism has dropped from 22% in 1985 to 2 to 6% in 1995–1997. During this period, the Reed Warblers’ rejection of non-mimetic model eggs has declined from rejection at 75% nests to 25% nests. Calculations suggest that this decline is too rapid to reflect genetic change in the host population and is more likely to reflect phenotypic flexibility. There is also a marked seasonal decline in rejection which matches the seasonal decline in parasitism frequency (Brooke et al. 1998). Some recent examples of rapid increases in host rejection which are correlated with increased parasitism (Takasu et al. 1993; Soler et al. 1994) may also reflect individual flexibility rather than genetic change through microevolution. Phenotypic flexibility in host responses will be favoured given the costs of rejection and small scale geographic variation and rapid temporal changes in parasitism rates (Lindholm 1999).
Stages in cuckoo-host co-evolution
These results suggest the following sequence of events for cuckoo-host co-evolution (Fig. 2). Some stages of this cycle have been modelled by Kelly (1987) and Takasu et al. (1993). The starting point is no host rejection and no cuckoo egg mimicry. In response to parasitism, hosts evolve rejection. In response to host rejection, cuckoos now evolve a mimetic egg. It could then pay hosts to accept the mimetic egg, provided there are sufficient costs of rejection and parasitism rates are not too high. The result is a stable equilibrium in the bottom left-hand box in Fig. 2. Alternatively, as the hosts evolve rejection it could pay the cuckoo to turn to new hosts and the cycle could start again. Once several hosts have evolved rejection, cuckoos will be forced to specialise on those hosts for whom their egg is a good match. Thus host-specialisation (separate gentes or species of cuckoos) is a likely outcome of co-evolution (Davies & Brooke 1989b).
Lanyon (1992) reached the opposite conclusion based on a molecular phylogeny of the parasitic cowbirds and related New World blackbirds (Icterinae). He found that species which are currently the most specialised in their use of hosts are the most ancient, with the two most generalist species being the most recently derived. So he concluded that specialist parasites evolve into generalists. However ‘number of hosts’ is unlikely to be a stable species characteristic. It could equally well be argued that because generalist parasites are younger then this represents the starting condition and that the older species have had more time to interact with hosts and to evolve specialisation.
WHY ARE SO MANY HOSTS ACCEPTORS OF NON-MIMETIC EGGS?
We now have a puzzle, namely why so many hosts of brood parasites lie in the top left hand box of Fig. 2 and show little rejection behaviour even to non-mimetic eggs. The famous example among hosts of the Common Cuckoo is the Dunnock (see above), which is one of the favourite hosts in Britain and which suffers similar parasitism rates to those hosts which have evolved rejection (Brooke & Davies 1987). There are many other acceptors among cuckoo hosts in Europe and Africa and also among cowbird hosts in North and South America.
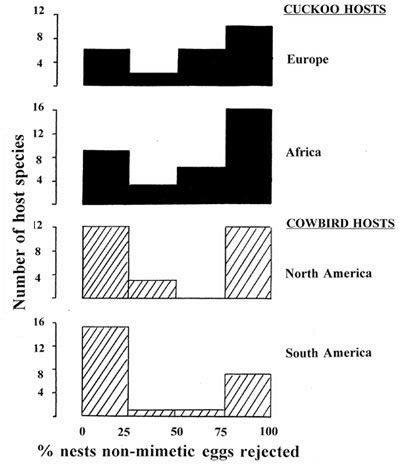
Fig. 3 updates the comparison first made by Rothstein (1990, 1992). Two problems with this comparison need to be admitted. First, as shown in the previous section, there is variation in rejection between host populations within a species in relation to parasitism frequency. The mean values per species in Fig. 3 are likely to conceal interesting variation which needs more study. Nevertheless, there are certainly marked differences between species. For example, there was good agreement between the measures of % rejection scored for sixteen suitable cuckoo host species studied both in Britain (Davies & Brooke 1989a) and Norway (Moksnes et al. 1991; rs = 0.818, P < 0.01). Second, different host species have different coloured eggs and different model eggs were used in different studies, so the term ‘non-mimetic’ is not quantified or standardised.
Despite these caveats, some interesting conclusions can be made. If this variation in host response to non-mimetic model eggs is categorised as species which are ‘acceptors’ (<25% rejection), ‘intermediate’ (26–75% rejection) and ‘rejectors’ (>75% rejection), there are significant differences in rejection frequency across the four continents (c26 = 12.97, P < 0.05). There are no differences in rejection by cuckoo hosts in Europe versus Africa (c22 = 0.34, P > 0.8), nor by cowbird hosts in North versus South America (c22 = 2.69, P > 0.20). The difference lies between the cowbird and cuckoo hosts. First, cowbird hosts show significantly less intermediate levels of rejection (5/51 species) than do cuckoo hosts (17/58 species; c21 = 5.26; P < 0.05). Second, there are more acceptors among cowbird hosts (53%, 27/51 species) than cuckoo hosts (26%, 15/58; c21 = 7.29, P < 0.01). A satisfactory account of host acceptance needs to explain these differences as well as the overall high levels of acceptance by hosts.Two hypotheses could explain the puzzle.
Hypothesis 1: Acceptor hosts would do better to reject. They accept because they are at the start of the co-evolutionary cycle.
Evolutionary lag may occur either because acceptor hosts lack the genetic variation which would permit rejection to evolve (Rothstein 1975b) or because selection takes a long time to change hosts from acceptors to rejectors when parasitism rates are low (Davies & Brooke 1989b). According to this hypothesis, host species on the left of Fig. 3 will, given time, move to the right. Four lines of evidence might support this view.
(a) There is good evidence for dynamic change in host–parasite systems. Brown-headed Cowbirds Molothrus ater have spread dramatically through North America during the last two hundred years, as forests have been cleared and agriculture has spread, and so must have come into contact with many new host species (Mayfield 1965; Robinson et al. 1995). Shiny Cowbirds Molothrus bonariensis have also recently spread north from South America to colonise the Caribbean (Post & Wiley 1977) and, in the last ten years, the South-eastern USA (Robinson et al. 1995). Cuckoo populations also show dynamic change, which may promote contact with new hosts. The Great Spotted Cuckoo Clamator glandarius in Spain (Arias de Reyna & Hidalgo 1982) and Common Cuckoo in Japan (Yamagishi & Fujioka 1986; Nakamura 1990) have recently begun to parasitise an apparently new host, the Azure-winged Magpie Cyanopica cyana.
(b) In some of these cases, the increase in parasitism has been associated with an increase in host rejection of non-mimetic eggs. For example, the Magpies Pica pica studied by Soler et al. (1994) in Spain increased rejection by 28% in nine years as parasitism by Great Spotted Cuckoos increased from 40% to 60% host nests. In Japan too, Azure-winged Magpies have been reported to show increased rejection over the last decade in response to increased parasitism by the Common Cuckoo (Takasu et al. 1993). However, these changes may not reflect evolution in the sense of genetic change (Zuniga & Redondo 1992; Lotem & Rothstein 1995). Similar rapid changes in host populations over time can reflect individual flexibility in defence tactics in response to changing benefits of rejection (Brooke et al. 1998).
(c) For many hosts of the Brown-headed Cowbird, calculations suggest that they would clearly do better to reject than to accept, given the heavy costs of parasitism (Rothstein 1975b). Even when all the likely costs of rejection are considered, their acceptance of eggs so different from their own set appears to be maladaptive (Rothstein 1982b). For hosts of the Common Cuckoo too, the variation in rejection of non-mimetic eggs (top graph in Fig. 3) is not obviously related to the current costs and benefits of rejection. For example, there is no relationship between the frequency of rejection and rates of parasitism, nor with the costs of rejection, as measured by abilities of hosts to eject eggs from their nests (Davies & Brooke 1989a).
(d) Finally, and perhaps most convincingly, Hosoi & Rothstein (1999) have expanded the original analysis on small samples by Mayfield (1965) to show that old hosts of the Brown-headed Cowbird (grassland and scrub species) have stronger rejection than new hosts (forest species, brought into recent contact with cowbirds through man’s creation of more ‘edge habitat’ between farmland and forest fringes). Even when controlling for the costs of cowbird parasitism (proportion of own young lost when a nest is parasitised), old hosts have stronger rejection. Furthermore, within the old hosts the variation in rejection is related to the costs of parasitism, with larger hosts, which suffer fewer losses from a cowbird chick in their nest, showing less rejection. However, within the new hosts, the variation in rejection is not related to costs. This is exactly what would be expected from evolutionary lag, with only old hosts having had sufficient time to evolve adaptations which reflect the costs and benefits of parasitism (Hosoi & Rothstein 1999).
Evolutionary lag could also explain the differences in rejection frequencies by hosts of cuckoos and cowbirds (Fig. 3). First, given the dramatic spread of cowbirds in recent history, it is likely that more cowbird hosts are new hosts, which could explain their greater proportion of acceptors. Second, parasitism levels of cowbird hosts tend to be much higher than those of cuckoo hosts, so selection for the evolution of rejection is often much stronger. This may explain why there are fewer cowbird hosts with ‘intermediate’ rejection. Once rejection appears in the host population, it sweeps rapidly through to fixation (Rothstein 1975b). Cuckoo hosts, with lower parasitism, may move more slowly from acceptance to rejection and so more may appear as intermediates in any one ‘snap shot’ of the evolutionary arms race (Davies & Brooke 1989a)
Hypothesis 2: Acceptor hosts do better to accept. Their acceptance reflects equilibrium conditions.
It is unlikely that all examples of acceptance will reflect evolutionary lag. Two lines of evidence support the idea that some or even total acceptance of non-mimetic eggs may be part of a stable outcome of co-evolution.
(a) In theory, we might not expect host populations to evolve to 100% rejection. This has been shown in an elegant analysis by Takasu et al. (1993) which combines a population genetic model for the evolution of a hypothetical ‘rejector gene’ with the population dynamics of an interaction between a specialist cuckoo and its host. The key point is that below a critical parasitism frequency, hosts do better to accept because of the costs of rejection (see previous section). Initially, when the host has no rejection, the cuckoo population increases. Rejection then spreads in the host population. As it does so, the cuckoo population declines and this may now result in parasitism dropping below the critical rate at which host rejection is favoured. The outcome may be an equilibrium with a mixture of rejector and acceptor hosts.
Takasu (1998) has extended his model to consider a generalist parasite, such as the Brown-headed Cowbird. Again, initially all the hosts are acceptors. As some hosts evolve rejection (those which suffer the greatest parasitism frequency or have the highest costs from parasitism) the cowbird population declines. This could then reduce parasitism pressure on the other hosts to below the critical rate for rejection to evolve. The stable outcome could be for most hosts to reject, a few to show intermediate levels of rejection and many others to accept. Takasu’s analysis could explain why the variation in rejection by hosts of the generalist parasites, the cowbirds, shows a stronger dichotomy into ‘acceptors’ and ‘rejectors’ than does the variation in rejection among hosts of specialist parasites, the cuckoos (Fig. 3).
(b) Empirical studies show that rejection of non-mimetic eggs can entail considerable costs so that acceptance could sometimes be the stable outcome (Lotem et al. 1995). Five recent studies support this view.
First, the learning mechanism used by hosts to recognise their own eggs (see previous section) may preclude the host population from evolving to 100% rejection. If a young bird is unlucky and is parasitised in its first breeding attempt, it will learn the foreign egg as part of its own set and will thereafter always accept it. This cost of misimprinting may explain why young birds are more likely to accept odd eggs in their nest (Lotem et al. 1995).
Second, because of the costs of rejection it pays to accept even non-mimetic eggs below a certain critical parasitism frequency (Davies et al. 1996; Brooke et al. 1998). Some of the variation in Fig. 3 could reflect different recognition costs for different hosts. For example, perhaps species with less variation in their own clutch are more likely to reject (Lotem & Nakamura 1998).
Third, among cowbird hosts, small-billed hosts are less able to eject the thick-shelled parasite egg. Their options are therefore either to desert or to accept. Acceptance may sometimes be the better of these two evils if there is a small payoff from desertion (e.g. because alternative nest sites scarce or because of a seasonal decline in food available) and if the hosts can raise some of their own young from a parasitised nest (Rohwer & Spaw 1998; Petit 1991).
Fourth, Brooker & Brooker (1996, 1998) have argued that Australian cuckoo hosts may be more likely to accept because the long breeding season reduces the costs of parasitism. In Australia, breeding seasons of 5 months are not unusual, which allows plenty of time for repeat nesting attempts after a failure. Pairs of Splendid Fairywrens Malurus splendens that have already raised young in a first brood, are less likely to breed again that season because the young remain on their natal territory at least until the following year, and there is an annual limit to the number of young that can be accommodated on the territory. A pair of wrens that accepts a cuckoo egg will raise none of their own young in the first attempt, but then still have time to catch up and raise as many young as non-parasitised pairs over the whole season. While this argument may explain why cuckoos are less costly than for species with short breeding seasons (most European hosts), it still leaves the puzzle of why the hosts don’t reject to save the bother of raising the cuckoo.
Finally, the costs of rejection could be imposed by the parasites themselves if they behaved like the Mafia and punished hosts that rejected by destroying their brood (Zahavi 1979). It is difficult to see how this would work for hosts of ejector cuckoos; they gain zero payoff from acceptance and surely would do better to desert and try again elsewhere, where there must at least be some possibility of escaping parasitism in the next attempt. However, for hosts that raise some of their own young in a parasitised nest, acceptance to avoid punishment could in principle bring greater reproductive success than rejection. Soler et al. (1995a) provide experimental evidence for this in Magpie Pica pica hosts of the Great Spotted Cuckoo.
Evolutionary lag or equilibrium?
With convincing theoretical arguments and empirical evidence for both hypotheses, the most likely conclusion is that the variation in nature will reflect a mixture of both systems at equilibrium and those at intermediate stages of a continuing arms race. Future studies need to bear both possibilities in mind when they assess the various costs and benefits of rejection by hosts. Perhaps the most convincing evidence for evolutionary lag would be to quantify the genetic differences between the different cuckoo gentes to test whether the least differentiated (and hence younger) gentes have the least discriminating hosts and the poorest mimicry by the cuckoo.
WHY DO HOSTS ACCEPT PARASITIC CHICKS?
An important message from recent studies is that it is not very useful to discuss the evolution of egg ‘rejection’ without specifying the mechanisms, because these will determine the costs and benefits involved. This applies equally to the problem of why hosts accept parasitic chicks.
Parasitic chicks are more likely to mimic the appearance of the host young in cases where they are raised alongside the host young than when they are raised alone (Davies & Brooke 1988). Lotem (1993) has suggested an ingenious explanation for this. When parasites are raised with host chicks, it pays hosts to learn the characteristics of the chicks in their nest (just as it pays them to learn about their eggs; see above). If they are unlucky, and are parasitised the first time they breed, they will imprint on both their own chicks and the parasite, and so will accept both in future attempts. But in most cases they will imprint only on their own young, and so will discriminate against odd-looking chicks in subsequent broods. This then selects for chick mimicry by the parasite. However, when the parasite is raised alone, this learning rule incurs a huge cost of misimprinting. If the host is parasitised in its first brood, it would imprint only on the parasite chick and would then reject its own young in future. In this case, Lotem showed that the rule ‘accept any chick in my nest’ does better than the learning rule. As predicted by this argument, hosts of the Common Cuckoo do indeed accept any foreign chick in their nest (Davies & Brooke 1988, 1989b). Experiments are now needed to test whether hosts of non-ejector brood parasites adopt the learning mechanism, and whether they discriminate against foreign chicks, as seems likely from the evidence presented by Nicolai (1964) for the estrildid hosts of Vidua parasites.
When parasites are raised alongside the host chicks, they may gain more food than the host young through superior competitive ability arising from their larger size (Lichtenstein & Sealy 1998), or through more selfish begging behaviour (Redondo 1993; Lichtenstein 1997), or through supernormal gape stimuli (Soler et al. 1995b). Greater selfishness in the parasitic chick is to be expected, given that it has no genetic stake in its nestmates (Harper 1986).
When parasites are raised alone, they do not face the problem of competition for food, only that of stimulating an adequate rate of host provisioning. At first sight, exaggerated begging is also expected because a lone parasite has no genetic interest in the host’s future reproduction. However, it may not pay to overstimulate the hosts if this means relying on a provisioning rate that the hosts have not evolved to sustain. This may explain why host provisioning rates to a lone cuckoo chick are about the same as for an average brood of their own young, even though in the short-term hosts can be stimulated to work at a higher rate if their brood size is increased (Brooke & Davies 1989; see also Gill 1982).
Although a single Common Cuckoo chick may need no special stimuli to induce the hosts to accept it (the hosts will accept any foreign nestling), our experiments reveal that its large size alone is not sufficient to stimulate adequate provisioning. Using Reed Warblers as hosts, we showed that single Blackbird Turdus merula, or Song Thrush, T. philomelos, chicks of the same mass as a cuckoo were fed at a lower rate. The Common Cuckoo chick’s key stimulus is its extraordinary rapid begging call (si-si-si-si) which sounds remarkably like a whole brood of host young, and which it matched in calling rate. When single Blackbird or Song Thrush chicks were accompanied by loudspeakers that broadcast Common Cuckoo begging calls, the hosts now fed them as much as a Common Cuckoo chick. Furthermore, broadcast of the calls of a brood of Reed Warblers had the same effect, supporting the idea that the Common Cuckoo’s call mimics a brood (Davies et al. 1998). The Common Cuckoo may need vocal trickery to stimulate adequate care because the hosts calibrate their provisioning rate both by what they see and by what they hear. Vocal mimicry of a brood may compensate for the fact that the Common Cuckoo presents a visual stimulus of just one gape.
Parasitic Honeyguides Indicator indicator, which use mandibular hooks to kill host nestlings, have also been reported to sound like several young (Fry 1974), so vocal mimicry of a brood may be widespread in cases where parasitic chicks are raised alone. Further experiments are needed to test whether the Common Cuckoo chick’s call is host specific, varying between the different gentes, or whether it simply mimics the general features of passerine brood calls, which would be effective for a range of hosts. In South Africa, the Diederik Cuckoo Chrysococcyx caprius has been reported to have different calls when raised by different hosts (Reed 1968). This raises the fascinating question of whether the calls are genetically programmed or develop through host parent conditioning to match the calls that are most effective in stimulating host care.
ACKNOWLEDGEMENTS
This review reports field work done together with Michael Brooke, Rebecca Kilner and David Noble, and I thank them, together with the Natural Environment Research Council who funded the research. I also thank Martin Baxter, Mike Cherry, Mike Lawes and Rob Slotow for comments on the manuscript.
REFERENCES
Arias de Reyna, L. & Hidalgo, S.J. 1982. An investigation into egg acceptance by Azure-winged Magpies and host recognition of Great Spotted Cuckoo chicks. Animal Behaviour 30: 819–823.
Baerends, G.P. & Drent, R.H. 1982. The Herring Gull and its eggs. Behaviour 82: 1–416.
Baker, E.C.S. 1942. Cuckoo Problems. London; Witherby.
Briskie, J.V., Sealy, S.G. & Hobson, K.A. 1992. Behavioural defences against avian brood parasitism in sympatric and allopatric host populations. Evolution 46: 334–340.
Brooke, M. de L. & Davies, N.B. 1987. Recent changes in host usage by Cuckoos Cuculus canorus in Britain. Journal of Animal Ecology 56: 873–883.
Brooke, M. de L. & Davies, N.B. 1988. Egg mimicry by Cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335: 630–632.
Brooke, M. de L. & Davies, N.B. 1989. Provisioning of nestling Cuckoos Cuculus canorus by reed warbler Acrocephalus scirpaceus hosts. Ibis 131: 250–256.
Brooke, M. de L. & Davies, N.B. 1991. A failure to demonstrate host imprinting in the Cuckoo Cuculus canorus and alternative hypotheses for the maintenance of egg mimicry. Ethology 89: 154–166.
Brooke, M. de L., Davies, N.B. & Noble, D.G. 1998. Rapid decline of host defences in response to reduced cuckoo parasitism: behavioural flexibility of reed warblers in a changing world. Proceedings of the Royal Society, B 265: 1277–1282.
Brooker, L. & Brooker, M. 1998. Why do Splendid Fairy-Wrens always accept cuckoo eggs? Behavioural Ecology 9: 420–424.
Brooker, M.G. & Brooker, L.C. 1989. The comparative breeding behaviour of two sympatric cuckoos, Horsfield’s Bronze-Cuckoo Chrysococcyx basalis and the Shining Bronze-Cuckoo C. lucidus, in western Australia: a new model for the evolution of egg morphology and host specificity in avian brood parasites. Ibis 131: 528–547.
Brooker, M.G. & Brooker, L.C. 1996. Acceptance by the Splendid Fairy-Wren of parasitism by Horsfield’s bronze-cuckoo: further evidence for evolutionary equilibrium in brood parasitism. Behavioural Ecology 7: 395–407.
Brooker, L.C., Brooker, M.G. & Brooker, A.M.H. 1990. An alternative population/genetics model for the evolution of egg mimesis and egg crypsis in cuckoos. Journal of Theoretical Biology 146: 123–143.
Chance, E.P. 1940. The Truth About the Cuckoo. London; Country Life.
Davies, N.B. & Brooke, M. de L. 1988. Cuckoos versus Reed Warblers: adaptations and counteradaptations. Animal Behaviour 36: 262–284.
Davies, N.B. & Brooke, M. de L. 1989a. An experimental study of co-evolution between the Cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. Journal of Animal Ecology 58: 207–224.
Davies, N.B. & Brooke, M. de L. 1989b. An experimental study of co-evolution between the Cuckoo, Cuculus canorus, and its hosts. II. Host egg markings, chick discrimination and general discussion. Journal of Animal Ecology 58: 225–236.
Davies, N.B., Brooke, M. de L. & Kacelnik, A. 1996. Recognition errors and probability of parasitism determine whether Reed Warblers should accept or reject mimetic cuckoo eggs. Proceedings of the Royal Society, B 263: 925–931.
Davies, N.B., Kilner, R.M. & Noble, D.G. 1998. Nestling cuckoos, Cuculus canorus, exploit hosts with begging calls that mimic a brood. Proceedings of the Royal Society, B 265, 673–678.
Duckworth, J.W. 1991. Responses of breeding Reed Warblers Acrocephalus scirpaceus to mounts of Sparrowhawk Accipiter nisus, Cuckoo Cuculus canorus and Jay Garrulus glandarius. Ibis 133: 68–74.
Freeman, S. 1988. Egg variability and conspecific nest parasitism in the Ploceus weaverbirds. Ostrich 59: 49–53.
Fry, C.H. 1974. Vocal mimesis in nestling Greater Honey-Guides. Bulletin of the British Ornithologists’ Club 94: 58–59.
Gärtner, K. 1982. Zur Ablehnung von Eiern und Jungen des kuckucks (Cuculus canorus) durch die Wirtsvögel: Beobachtungen und experimentelle Untersuchungen an Sumpfrohrsänger. Vogelwelt 103: 201–224.
Gaston, A.J. 1976. Brood parasitism by the Pied Crested Cuckoo Clamator jacobinus. Journal of Animal Ecology 45: 331–345.
Gibbs, H.L., Brooke, M. de L. & Davies, N.B. 1996. Analysis of genetic differentiation of host races of the Common Cuckoo Cuculus canorus using mitochondrial and microsatellite DNA variation. Proceedings of the Royal Society, B 263: 89–96.
Gill, B.J. 1982 The Grey Warbler’s care of nestlings: a comparison between unparasitised broods and those comprising a Shining Bronze-Cuckoo. Emu 82: 177–181.
Harper, A.B. 1986. The evolution of begging: sibling competition and parent–offspring conflict. American Naturalist 128: 99–114.
Higuchi, H. 1989. Responses of the Bush Warbler Cettia diphone to artificial eggs of Cuculus cuckoos in Japan. Ibis 131: 94–98.
Hosoi, A. & Rothstein, S.I. 1999. The enigma of nest desertion: is it really a defense against brood parasitism? Animal Behaviour, in press.
Kelly, C. 1987. A model to explore the rate of spread of mimicry and rejection in hypothetical populations of cuckoos and their hosts. Journal of Theoretical Biology 125: 283–299.
Lanyon, S.M. 1992. Interspecific brood parasitism in Blackbirds (Icterinae): a phylogenetic perspective. Science 255: 77–79.
Lawes, M.J. & Kirkman, S. 1996. Egg recognition and interspecific brood parasitism rates in Red Bishops (Aves: Ploceidae). Animal Behaviour 52: 553–563.
Lichtenstein, G. 1997. Begging behaviour and host manipulation by three species of parasitic cowbirds. PhD thesis, University of Cambridge, UK
Lichtenstein, G. & Sealy, S.G. 1998. Nestling competition, rather than supernormal stimulus, explains the success of parasitic Brown-Headed Cowbird chicks in Yellow Warbler nests. Proceedings of the Royal Society, B 265: 249–254.
Lindholm, A.K. 1997. Evolution of host defences against avian brood parasitism. PhD thesis, University of Cambridge, UK
Lindholm, A.K. 1999. Brood parasitism by the cuckoo on patchy reed warbler populations in Britain. Journal of Animal Ecology, in press.
Lindholm, A.K. & Thomas, R.J. 1999. Differences between populations of reed warblers in defences against brood parasitism. Behaviour, in press.
Lotem, A. 1993. Learning to recognize nestlings is maladaptive for Cuckoo Cuculus canorus hosts. Nature 362: 743–745.
Lotem, A., Nakamura, H. & Zahavi, A. 1995. Constraints on egg discrimination and cuckoo-host co-evolution. Animal Behaviour 49: 1185–1209.
Lotem, A. & Nakamura, H. 1998. Evolutionary equilibria in avian brood parasitism: an alternative to the ‘arms race – evolutionary lag’ concept. In: Rothstein, S.I. & Robinson, S.K. (eds) Parasitic birds and their hosts. Oxford University Press.
Lotem, A. & Rothstein, S.I. 1995 Cuckoo-host coevolution: from snapshots of an arms race to the documentation of microevolution. Trends in Ecology and Evolution 10: 436–437.
Marchetti, K. 1992. Costs to host defence and the persistence of parasitic cuckoos. Proceedings of the Royal Society B 248: 41–45.
Mayfield, H. 1965. The Brown-Headed Cowbird, with old and new hosts. Living Bird 4: 13–28.
Moksnes, A. & Røskaft, E. 1989. Adaptations of meadow pipits to parasitism by the Common Cuckoo. Behavioural Ecology and Sociobiology 24: 25–30.
Moksnes, A. & Røskaft, E. 1995. Egg morphs and host preference in the Common Cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. Journal of Zoology 236: 625–648.
Moksnes, A., Røskaft, E., Braa, A.T., Korsnes, L., Lampe, H.M. & Pedersen, H.Ch. 1991. Behavioural responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour 116: 64–89.
Moksnes, A., Røskaft, E. & Korsnes, L. 1993. Rejection of Cuckoo (Cuculus canorus) eggs by Meadow Pipits (Anthus pratensis). Behavioural Ecology 4: 120–127.
Nakamura, H. 1990. Brood parasitism by the Cuckoo Cuculus canorus in Japan and the start of new parasitism on the Azure-Winged Magpie Cyanopica cyana. Japanese Journal of Ornithology 39: 1–18.
Nicolai, J. 1964. Der Brutparasitismus der Viduinae als ethologisches Problem. Zeitschrift für Tierpsychologie 21: 127–204.
Noble, D.G. 1995. Coevolution and ecology of seven sympatric cuckoo species and their hosts in Namibia. PhD thesis, University of Cambridge, UK
Øien, I.J., Moksnes, A. & Røskaft, E. 1995. Evolution of variation in egg colour and marking pattern in European passerines: adaptations in a coevolutionary arms race with the Cuckoo Cuculus canorus. Behavioural Ecology 6: 166–174.
Payne, R.B. & Payne, K. 1967. Cuckoo hosts in southern Africa. Ostrich 38: 135–143.
Petit, L.J. 1991. Adaptive tolerance of cowbird parasitism by prothonotary warblers: a consequence of nest-site limitation? Animal Behaviour 41: 425–432.
Post, W. & Wiley, J.W. 1977. Reproductive interactions of the Shiny Cowbird and the Yellow-Shouldered Blackbird. Condor 79: 176–184.
Redondo, T. 1993. Exploitation of host mechanisms for parental care by avian brood parasites. Etologia 3: 235–297.
Reed, R.A. 1968. Studies of Diederik Cuckoo Chrysococcyx caprius in the Transvaal. Ibis 110: 321–331.
Robinson, S.K., Rothstein, S.I., Brittingham, M.C., Petit, L.J. & Grzybowski, J.A. 1995. Ecology and behavior of cowbirds and their impact on host populations. In: Martin, T.E. & Finch, M. (eds). Ecology and Management of Neotropical Migratory Birds. Oxford University Press.
Rohwer, S. & Spaw, C.D. 1988. Evolutionary lag versus bill-size constraints: a comparative study of the acceptance of cowbird eggs by old hosts. Evolutionary Ecology 2: 27–36.
Rothstein, S.I. 1974. Mechanisms of avian egg recognition: possible learned and innate factors. Auk 91: 796–807.
Rothstein, S.I. 1975a. Mechanisms of avian egg recognition: do birds know their own eggs? Animal Behaviour 23: 268–278.
Rothstein, S.I. 1975b. Evolutionary rates and host defences against avian brood parasitism. American Naturalist 109: 161–176.
Rothstein, S.I. 1978. Mechanisms of avian egg recognition: additional evidence for learned components. Animal Behaviour 26: 671–677.
Rothstein, S.I. 1982a. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejector species? Behavioural Ecology and Sociobiology 11: 229–239.
Rothstein, S.I. 1982b. Successes and failures in avian egg recognition with comments on the utility of optimality reasoning. American Zoologist 22: 547–560.
Rothstein, S.I. 1990. A model system for coevolution: avian brood parasitism. Annual Review of Ecology and Systematics 21: 481–508.
Rothstein, S.I. 1992. Brood parasitism, the importance of experiments and host defences of avifaunas on different continents. Proceedings of the VII Pan-African Ornithological Congress, 521–535.
Rowan, M.K. 1983. The doves, parrots, louries and cuckoos of southern Africa. Croom Helm, London.
Seel, D.C. 1977. Migration of the northwestern European population of the Cuckoo, Cuculus canorus, as shown by ringing. Ibis 119: 309–322.
Soler, J.J. & Møller, A.P. 1996. A comparative analysis of the evolution of variation in appearance of eggs of European passerines in relation to brood parasitism. Behavioural Ecology 7: 89–94.
Soler, M., Soler, J.J., Martinez, J.G. & Møller, A.P. 1994. Micro-evolutionary change in host response to a brood parasite. Behavioural Ecology and Sociobiology 35: 295–301.
Soler, M., Soler, J.J., Martinez, J.G. & Møller, A.P. 1995a. Magpie host manipulation by great spotted cuckoos: evidence for an avian Mafia? Evolution 49: 770–775.
Soler, M., Martinez, J.G., Soler, J.J. & Møller, A.P. 1995b. Preferential allocation of food by Magpies Pica pica to Clamator glandarius chicks. Behavioural Ecology and Sociobiology 37: 7–13.
Soler, M., Soler, J.J. & Martinez J.G. 1997. Great Spotted Cuckoos improve their reproductive success by damaging Magpie host eggs. Animal Behaviour 54: 1227–1233.
Takasu, F. 1998. Why do all host species not show defense against avian brood parasitism: evolutionary lag or equilibrium? American Naturalist 151: 193–205.
Takasu, F., Kawasaki, K., Nakamura, H., Cohen, J.E. & Shigesada, N. 1993. Modelling the population dynamics of a cuckoo-host association and the evolution of host defenses. American Naturalist 142: 819–839.
Victoria, J.K. 1972. Clutch characteristics and egg discriminative ability of the African village weaverbird Ploceus cucullatus. Ibis 114: 367–376.
Wyllie, I. 1981. The Cuckoo. London: Batsford.
Yamagishi, S. & Fujioka, M. 1986. Heavy brood parasitism by the Common Cuckoo Cuculus canorus on the Azure-Winged Magpie Cyanopica cyana. Tori 34: 91–96.
Zahavi, A. 1979. Parasitism and nest predation in parasitic cuckoos. American Naturalist 113: 157–159.
Zuniga, J.M. & Redondo, T. 1992. No evidence for variable duration of sympatry between the Great Spotted Cuckoo and its Magpie host. Nature 359: 410–411.
Table 1. When a Common Cuckoo, Cuculus canorus, parasitises a Reed Warbler nest which already contains a Cuckoo egg (either a real one or a model egg), is it more likely to remove the Cuckoo egg rather than a host egg?

1. Data in Table V of Davies & Brooke 1988.
2. Reed Warbler models with green spots. Two more cases added to the eight cases in Table VI op.cit.
3. Pied Wagtail, Redstart, Meadow Pipit and Reed Warbler rufous spot models. Four more cases added to the five in Table VI op.cit.
4. Where there was uncertainty about the exact number of eggs in the nest at the time of parasitism (because nests were not checked every day), averages are given, hence some ‘half eggs’ in the total column. On the 21 cases where the contents were known, in 18 cases the cuckoo removed one egg, and in three cases it removed two eggs.
5. Binomial model with normal asymptotics to test whether cuckoo eggs are more likely to be removed than a Reed Warbler egg, hence one-tailed test.
Fig. 1. The cycle of co-evolution between cuckoo and host.
Fig. 2. Stages of cuckoo-host co-evolution suggested by observations and experiments described in the text.
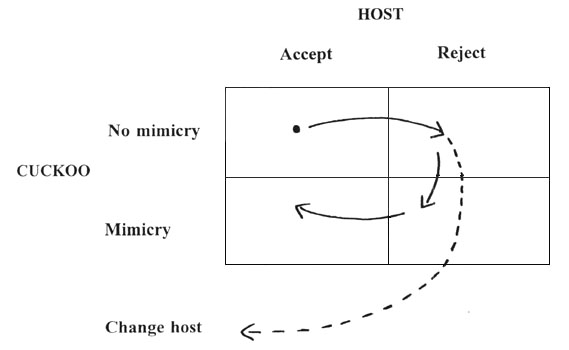
Fig. 3. Rejection of non-mimetic eggs by suitable hosts of brood parasites on four continents, updating the comparison first made by Rothstein (1992). The histograms show the frequency distribution of the number of species with various rejection frequencies, measured as % nests from which model eggs were rejected. Data from following sources: 24 hosts of Cuckoo Cuculus canorus in Europe (Davies & Brooke 1989a; Moksnes et al. 1991; means per species): 34 hosts of Cuculus, Chrysococcyx, Oxylophus and Clamator cuckoos in South Africa and Namibia (Noble 1995; Lawes & Kirkman 1996; Lindholm 1997): 27 hosts of Molothrus ater and M. aeneus in North America (Rothstein 1992): 24 hosts of M. bonariensis in South America (Rothstein 1992).