S11.1: Hatching patterns in nonprecocial birds: A preliminary comparative analysis
Javier Viñuela & Luis María Carrascal
Departamento de Ecología Evolutiva, Museo Nacional de Ciencias Naturales, José Gutierrez Abascal 2, 28006 Madrid, Spain, fax 34-91-5645078, e-mail mcnv109@fresno.csic.es
Viñuela, J & Carrascal, L.M. 1999. Hatching patterns in nonprecocial birds: A preliminary comparative analysis. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
584-599. Johannesburg: BirdLife South Africa.Hatching asynchrony and incubation onset were analysed in 117 nonprecocial species by phylogenetic autocorrelation. Phylogeny had a significant effect on clutch size, type of nest, hatching asynchrony, preincubation period, body size, and predation rate, but not on latitude. Both hatching asynchrony and preincubation period were positively correlated with clutch size. Birds started incubation earlier at lower latitudes, but no latitudinal variation in hatching asynchrony was found. Neither predation rate nor nest type (open, dome or closed nests) affected hatching asynchrony or the onset of incubation. Birds may start incubation earlier in hot climates of lower latitudes to preserve egg viability. Latitudinal variation in clutch size may be partly explained by earlier onset of incubation at low latitudes that may impose a constraint on hatching asynchrony. The occurrence of brood reduction, type of diet, mode of incubation (only female- vs. both sexes), mating system and social system were unrelated to the onset of incubation. However, hatching asynchrony was larger in species feeding on plant food and in carnivores than in omnivorous or insectivorous birds. Social species tended to have more asynchronous hatching than solitary breeders. These results support the ‘offspring quality assurance’ and ‘brood parasitism’ hypotheses. Birds seem to maintain a balance between hatching asynchrony and incubation delay as clutch size increases, probably due to the effect of several simultaneous, opposing selective pressures.
INTRODUCTION
The evolutionary significance of hatching asynchrony in altricial birds has been strongly debated during the last decades (Stoleson & Beissinger 1995). Hatching asynchrony could be a mechanism to establish an intra-brood size hierarchy that allows a quick death of smaller, last-hatched chicks, when there is not enough food to raise all chicks (the ‘brood reduction’ hypothesis; Lack 1954; Ricklefs 1965). Clark & Wilson (1981) and Hussell (1972, 1985), challenged that interpretation, and proposed that hatching asynchrony was an adaptation to minimise brood losses due to predation (‘nest failure’ hypothesis). An early onset of incubation, promoting hatching asynchrony, reduces the total time early-laid eggs are exposed to predators, so it may increase the probability of raising some chick to fledging.
Clark & Wilson (1981) examined the hatching patterns of 87 species of altricial birds. Their results suggested that nest-failure was more important than brood reduction in driving asynchronous hatching. However, Slagsvold (1986) criticised Clark & Wilson’s (1981) comparative analysis because it was not clear how hatching asynchrony varied with nesting habits (open- vs. hole-nesters), geographic region (from tropical to arctic regions), or clutch size. All of these factors could be related to predation pressure. Furthermore, Clark & Wilson (1981) classified hatching patterns categorically based on starting incubation with the first-laid egg (1) to starting incubation with the last-laid egg (N). Using this method, a tropical species would have a hatching asynchrony of N-1 by starting incubation with the first egg with a clutch size of two, which is the common in tropics. Temperate species with much larger clutches would also be classified as N-1, but would start incubation on the 6th egg (see Slagsvold 1986).
There were two other shortcomings of the comparative analysis performed by Clark & Wilson (1981). First, Passerines, a group where hatching asynchrony is usually low, were overrepresented (Stoleson & Beissinger 1995). Second, the possible effect of phylogeny on hatching patterns was not considered. Species are not independent units, as they share ancestors at different levels of their phylogeny (Felsenstein 1985; Harvey & Pagel 1991). Forgetting this fact may lead to errors in the interpretation of the ecological significance of the characters analysed (Harvey 1996), especially when evolutionary history explains a high percentage of the phenotypic variability observed among extant species (Harvey & Pagel 1991; Miles & Dunham 1993; Martins & Hansen 1996).
The comparative analyses by Clark & Wilson (1981) still remains unique. Their work stimulated much research, and as many as 17 alternative hypothesis to explain hatching asynchrony have been proposed (review in Stoleson & Beissinger 1995; see also Veiga 1993 and Slagsvold et al. 1995). It is increasingly clear that no single hypothesis can explain variation in hatching asynchrony among or even within a species. Nevertheless, the relative importance of different hypotheses is unknown (Stoleson & Beissinger 1995). Stoleson & Beissinger (1995) suggested the need for a comprehensive comparative analysis considering the possible effects of phylogeny.
In this paper, we present a preliminary comparative analysis. We acknowledge that additional work will be necessary to discard or support the many hypotheses proposed to explain hatching patterns in birds, by analysing more variables, species and environmental conditions. We use many of the factors considered by Clark & Wilson (1981), such as latitude, nesting habits, clutch size, and predation rate. We also explore the possible effect of other factors that could be related to hatching patterns, such as hatching success, food habits, incubation roles, brood reduction, sibling aggression and mating system.
METHODS
Data sources
We performed an extensive review of the available literature on hatching asynchrony and breeding ecology of birds. The starting point was the bibliographic data bases provided by Clark & Wilson (1981), Koenig (1982), and Stoleson & Beissinger (1995). Additional literature searching was performed in the Zoological Record and Orbis bibliographic data bases, using the key words ‘hatching asynchrony’, ‘breeding ecology’ and ‘breeding biology’. We used original sources to estimate, as accurately as possible, the location of the study area. Due to space limitations, we are unable to present the list of species here (but it is available from the authors upon request). The data base include 117 nonprecocial species from 44 families. The following orders were represented: Sphenisciformes (3 species), Pelecaniformes (4), Ciconiiformes (7), Falconiformes (10), Charadriiformes (4), Columbiformes (5), Psittaciformes (3), Cuculiformes (3), Strigiformes (3), Caprimulgiformes (3), Apodiformes (4), Coliiformes (1), Coraciiformes (6), Piciformes (1), and Passeriformes (60).
Definition of variables
For each species we noted the latitude of the study area. When data were from large areas, we considered the average latitude of the study area. Mean clutch size and nest type (open, closed or dome) were noted for each species. Whenever provided, the laying interval in days between laying of successive eggs was noted. This trait appears to vary little and often was not reported. When it was missing, we used the values from the most taxonomically similar species, which was usually one day for Passerines and other small size birds, two days for mid-size birds, and three days for larger species.
Hatching asynchrony was defined as the numbers of days elapsed between hatching of the first and last chicks in a brood for the average clutch size. In species with synchronous hatching, we used a value of 0.5 days whenever a value for the hatching period was not given, since even in the most synchronous species the hatching period is rarely less than 12 hours (Stoleson & Beissinger 1995).
Hatching asynchrony is the consequence of birds starting incubation before the end of the laying period. Stoleson & Beissinger (1995) remarked that the factors affecting the onset of incubation could be different from those related to hatching asynchrony. Consequently, we have considered the preincubation period, defined as the number of days elapsed between laying of the first egg and the onset of incubation, for the average clutch size of the species. This variable was missing in about 60 % of the species considered. In such cases, we estimated the preincubation period by backdating from hatching asynchrony, assuming that hatching patterns mainly reflect incubation patterns (see Clark & Wilson 1981). For example, in a species with an average clutch size of six eggs, laying interval of 1 day, and hatching asynchrony of 2 days, preincubation period was estimated to be 3 days.
Body mass of adult birds for each species was taken from original sources, whenever provided, or from Dunning (1996). The average of male and female body masses were considered for species that are sexually dimorphic in size.
When provided, the percentage of eggs and nestlings lost to predators was noted. More often, an overall percentage of nest loss was found. Four categories of food habits were considered: plant food (herbivorous, granivorous, frugivorous and nectarivorous), omnivorous (plant and animal food), insectivorous, and other animal food (carnivores and fish-eaters). Species were classified as those where only the female incubates, or both sexes incubate. We also noted whether or not brood reduction (nestlings deaths by starvation or siblicide) and sibling aggression occurred. We distinguished between apparently monogamous and polygynous species. Four categories of social systems were considered: species with type I territories, colonial, gregarious but not colonial, and other (mainly solitary breeders that do not clearly defend a territory).
Hatching asynchrony and preincubation period data were found for 319 species. However, some variables, especially predation rate, were often not reported. Partial predation rates on eggs and chicks were even harder to find. This factor was considered essential in the comparative analysis of Clark & Wilson (1981). Consequently, the final sample includes only 117 species of nonprecocial birds (altricial, semi-altricial and semi-precocial) for which data of overall predation rate could be obtained. An adequate test of Clark & Wilson’s ideas would require detailed information on partial predation rates during the laying and fledging periods (see Hussell 1985). This information is rarely reported and difficult to obtain. However, examining latitudinal trends in hatching asynchrony, or the relationship between overall predation rate or nesting habits and hatching asynchrony, is an indirect method that may be used to test the nest failure hypothesis (Clark & Wilson 1981).
Phylogenetic analysis
Working with great numbers of species and a sufficiently well-known phylogenetic hypothesis (Martins & Hansen 1996), it is possible to use the phylogenetic autocorrelation method (Cheverud et al. 1985; Gittleman & Kot 1990). This method estimates the percentage of variance in traits explained by the phylogenetic hypothesis, and partitions the observed variability into a phylogenetic component and another specific component that is not due to common ancestry. If the goal of analyses is to estimate the relationships between variables in ecological time to test hypotheses related to selection pressures (e.g., analysis by Clark & Wilson 1981), then the effect of common ancestry on traits should be estimated and removed. Therefore, all analyses were performed using the residuals of phylogenetic autocorrelation method.
Data were analysed with the phylogenetic first order autoregressive method (Cheverud et al. 1985; Gittleman & Kot 1990). The autoregressive method partitions the phenotypical variance of a character (y) in a component that is attributable to the phylogenetic relatedness of the species (phylogenetic component, Wy) and another nonphylogenetic component attributable to the independent evolution of each species (specific component, e ; y = r Wy + e ). This method provides the autocorrelation coefficient (r ), which measures the correlation between the phenotypic trait y and the purely phylogenetic prediction Wy. The phylogenetic component is a prediction of trait values for each species based solely on relatives of varying relatedness. The specific component e represents the portion of each trait unaccounted for by interspecific (phylogenetic) relationships. This method is quite robust in terms of the uncertainties in the length of the branches of the phylogenetic tree for well known topologies (Purvis et al. 1994; Martins & Hansen 1996).
The matrix of phylogenetic relatedness (W) of n x n species summarises the phylogenetic distances between the species studied (n = 117). The phylogenetic hypothesis used was taken from Sibley & Ahlquist (1990), based on DNA-DNA hybridisation data. This work provides the only topology for all superfamilies, families, subfamilies, tribes and genera used in this study, and is fairly well resolved (Mooers & Cotgrave 1994; but see Sarich et al. 1989). D T50H values provided by Sibley & Ahlquist (1990) were used to build the matrix of phylogenetic relatedness among species. We used six levels of phylogenetic distances considering D T50H values: 25, 20, 15, 10, 5, 1. We worked with topologies resolved up to the level of genus, due to the fact that our sampling unit is the species and there is no topological definition for all of the species studied below this taxonomic level. Thus, the weights of the matrix W (wij; the weight assigned to species j in computing the value of species i) are functions of the phylogenetic relatedness of the species included in the analysis to each other using a hierarchical distance based on systematic-phylogenetic affinity (see Jordano 1995 for a similar approach). To improve model fit, we used the grid search procedure for maximum likelihood estimator described by Gittleman & Kot (1990) to derive wij values. Using this method, the form of the decreasing function for phylogenetic connectivity values with increasing phylogenetic distance need not be assumed a priori, unlike the method of Cheverud et al. (1985).
The statistical package by J.L. Gittleman and H. Luh, including programs AUTOSEARCH.EXE and AUTOMORAN.EXE, was used to build the connectivity matrix W, and to estimate r and R2 (the variance explained by the phylogenetic hypothesis) using a maximum likelihood procedure. See Cheverud et al. (1985), Gittleman & Kot (1990) and Gittleman et al. (1996) for computational details. Higher r values indicate that related species tend to be similar in morphological/ecological traits. All variables were log(e)-transformed prior to the analysis. Residuals from the autoregressive model were tested for independence following Gittleman & Kot (1990).
Statistical analyses
Residuals of phylogenetic autocorrelation method were analysed by means of multiple linear regressions. Since the mean of phylogenetic residuals is zero, multiple regressions were performed without estimating the intercept. Categorical variables (food habits, incubation roles, brood reduction, sibling aggression, mating system, and social system) could not been analysed using phylogenetic autocorrelation method, because these variables followed binomial distributions or could not be linearized. Nevertheless, categorical variables were included in ANCOVA models, using the same dependent and independent variables included in multiple regression analyses. Independent variables were used as covariates. This approach tested the influence of each variable, controlling for the effects of covariates. We realise that these analyses did not control for the effects of phylogeny, and therefore the distorting effects of non-adaptive variations in biological traits are unknown (e.g., cladogenetic processes).
Raw data, and phylogenetic hypothesis used with the phylogenetic autocorrelation method, are available upon request by e-mail.
RESULTS
Phylogenetic effects
Phylogenetic autoregressive analyses revealed significant autocorrelations in seven of eight variables (Fig. 1), suggesting that common ancestry had a significant effect in observed phenotypic variation. Body mass showed the highest phylogenetic effect and hatching asynchrony showed the second highest, followed by nest type, clutch size, and the percentage of eggs and nestlings lost to predators. Preincubation period showed significant phylogenetic effects of <10%. Latitude was the only variable with a nonsignificant phylogenetic effect.
Analyses of continuous variables using phylogenetic residuals
The length of preincubation period was typically between 0 and 2 days (Fig. 2A). Interspecific variation in the length of the preincubation period not attributable to common ancestry (i.e. phylogenetic residuals) was significantly explained by the variables in Table 1 (F5,112 = 11.479, P < 0.001; R2= 33.9%). Length of preincubation period was significantly and positively related to mean clutch size and latitude. Interspecific variation in the preincubation period was mainly explained by clutch size (Table 1). In summary, removing the effect of evolutionary history on present-day variation in variables, longer preincubation periods were observed in species with larger clutches breeding at higher latitudes.
Asynchrony in hatching was typically also between 0 and 2 days (Fig. 2B). Hatching asynchrony was significantly predicted by variables in Table 2 (F5,112 = 5.983, P < 0.001; R2 = 21.1%). Hatching asynchrony was greater in larger species and was positively related to clutch size, which was the main variable explaining non-phylogenetic interspecific variation.
Mean clutch size was significantly and highly explained by variables in Table 3 (F6,111 = 36.124, P < 0.001; R2= 66.1%) after removing the effect of evolutionary history on present-day variation in variables. Large clutch sizes were significantly associated with small-sized species, with cavity nesters, with longer preincubation periods, and with higher hatching asynchronies. Preincubation period and hatching asynchrony accounted for the largest proportion in variance explained in mean clutch size.
Other factors affecting the onset of incubation and hatching asynchrony
Preincubation period was not significantly (P > 0.1) related to brood reduction, sibling aggression, food habits, incubation roles, mating system or social system when these factors were included in ANCOVA analyses using latitude and clutch size as covariates.
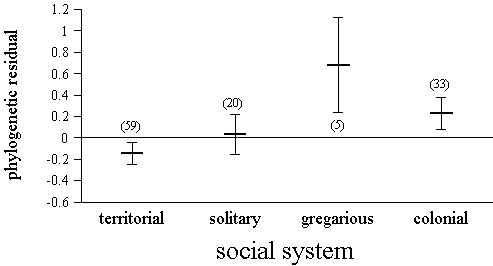
ANCOVA analyses performed with hatching asynchrony as the dependent variable, and adult body mass and clutch size as covariates, only found significant effects for food habits (F3,111 = 4.28, P = 0.007) and social system (F3,111 = 3.13, P = 0.028). Species feeding on plant food had the largest hatching asynchronies, followed by carnivorous species, while insectivorous and omnivorous species had more synchronous hatching (Fig. 3). Territorial species tended to have more synchronous hatching than predicted, while social birds tended to have large hatching asynchronies (Fig. 4).
DISCUSSION
Latitudinal variation in hatching asynchrony and preincubation period.
Our results show that birds tend to start incubation earlier at low latitudes, even after removing the effects of phylogeny and clutch size, and after considering the possible effects of other factors, such as body mass or nest type. In contrast, no significant effect of latitude on hatching asynchrony was detected. These results suggest that there is a relationship between latitude and the onset of incubation, but not between latitude and hatching asynchrony. An advance in the onset of incubation in hot climates of low latitudes is predicted by the egg viability hypothesis (Arnold et al. 1987; Veiga 1992; Veiga & Viñuela 1993; Stoleson & Beissinger 1995, 1997a), because unincubated eggs lose viability more quickly as temperatures approach ‘physiological zero’ (24-27o C; Webb 1987; Veiga 1992; Arnold 1993; Veiga & Viñuela 1993; Stoleson 1996, 1999). Thus, birds should tend to start incubation earlier where temperatures are higher (i.e. at low latitudes). Our results support Arnold’s (1993) findings for egg viability and clutch size among North American Anatidae.
The nest failure hypothesis also predicts an earlier onset of incubation in tropical latitudes, if we assume that predation pressure is higher in the tropics. However, we found no relationship between predation rate and hatching asynchrony or preincubation period in our sample of 117 species. Also, we did not find any correlation between nest failure rate and latitude (without considering phylogeny: r = - 0.07, P = 0.43; considering phylogeny: r = -0.08, P = 0.39). Some recent experimental work also challenges the ‘classical’ view of higher predation pressure in the tropics (Tellería & Díaz 1995). We found a highly significant correlation between nest failure rate and nest type (without phylogeny: r = - 0.38, P < 0.001; considering phylogeny: r = - 0.25, P = 0.006), suggesting that predation pressure is higher among open nesters that in hole nesters (see Clark & Wilson 1981 and references therein). However, hatching asynchrony and preincubation period did not significantly vary with nest type. In conclusion, the latitudinal variation in preincubation period seems unlikely to be due to predation pressure. This raises questions about the results of Clark & Wilson (1981) as discussed by Slagsvold (1986).
The ‘energy constraints hypothesis’ (Nilsson 1993a) also would predict an earlier onset of incubation in the tropics, assuming that energy expenses of incubation are lower at low latitudes. However, we did not find a difference in preincubation period between species where only the female incubates and those were both sexes share incubation duties, a clear prediction of this hypothesis (Nilsson 1993a; Stoleson & Beissinger 1995). This hypothesis could at least partially explain incubation and hatching patterns in small passerines (Nilsson 1993b), but may not apply to larger species where energy demands of incubation or laying are low (see Viñuela 1997).
Latitudinal variation in clutch size
The main factor related to hatching asynchrony and preincubation period in our analysis was clutch size. Larger clutch sizes not only had longer hatching asynchronies, but also longer preincubation periods. We could view the variation in hatching asynchrony and incubation onset as a consequence of the variation in clutch size. Long preincubation delays and large hatching asynchronies may require large clutch sizes. However, the opposite could also be true: clutch size could be adjusted to constraints posed by hatching asynchrony or the onset of incubation (Stoleson 1999). Clutch size in birds typically varies with latitude, tropical species having smaller clutches than birds at higher latitudes (review in Murray 1985), although our data did not find this trend. It has been suggested that latitudinal variation in clutch size could be a consequence of constraints on egg viability (Arnold 1993; Stoleson & Beissinger 1995; Stoleson 1999): birds in the tropics might start incubation earlier, so having large clutches there would result in large hatching asynchronies (e.g., Beissinger & Waltman 1991). Given that mortality induced by hatching asynchrony could often be a non-adaptive consequence of hatching asynchrony (Amundsen & Slagsvold 1991b; Stoleson & Beissinger 1997b), one strategy in the tropics would be to have lower clutch sizes.
Birds may balance between incubation onset and hatching asynchrony, because most species tend to have hatching patterns (or preincubation delays) intermediate between the maximal and minimal possibilities. From the extensive experimental work done during last two decades, it has become increasingly clear that several selective pressures could act on hatching asynchrony simultaneously in a given species. Some of these pressures could favour an early onset of incubation, others might select for a small hatching asynchrony (Clark & Wilson 1981, 1985; Bollinger et al. 1990; Viñuela 1991; Veiga & Viñuela 1993; see a model for the House Sparrow in Stoleson & Beissinger 1995). If this was the case, we should expect to find intermediate patterns of asynchrony. This prediction was partly supported by our results (Fig. 2), although the majority of species hatched their eggs <2 days apart.
Our results show that clutch size was higher in closed nesters than for open nesters, as previously reported in numerous studies (see reviews in Martin 1992, 1995), but that clutch size was not related to predation rate (albeit predation rate clearly varied with nesting habits). This gives support to the recently proposed ‘limited breeding opportunities’ hypothesis (Beissinger & Waltman 1991; Martin 1993; Beissinger 1996), because clutch size was related to nesting habits, but not to predation. Clutch size did not significantly increase with latitude and was negatively correlated with body size when hatching asynchrony and preincubation period were included as independent variables in a multiple regression analysis. When we removed hatching asynchrony from this model, the relationship between clutch size and latitude remained practically unchanged (beta = 0.113 ± 0.084 [SE], see beta in Table 3; P >0.2 in t-test comparing standardised regression coefficients). However, when preincubation period was removed, the effect of latitude on clutch size became significant (beta = 0.279 ± 0.081, P < 0.001), and the slope of the linear regression changed significantly (t223= 2.17, P = 0.031 in t-test comparing standardised regression coefficients). There could be a latitudinal trend in birds to start incubation earlier and, at the same time, to reduce clutch size, and this would explain why we did not find any effect of latitude on hatching asynchrony.
Other factors affecting hatching asynchrony or incubation onset
Preincubation period was not affected by other variables considered. Neither brood reduction, sibling aggression, food habits, distribution of incubation duties, mating system, or social system were significant when the effect of phylogeny was removed.
We found a significant effect of food habits on hatching asynchrony. Carnivorous species and those eating plant food tended to have higher hatching asynchronies than omnivorous or insectivorous species. Food habits could explain or be explained by the relationship between hatching asynchrony and body size. When food habits were considered in an ANCOVA analysis, the correlation with body size disappeared. Body size clearly varied among food habits (ANOVA without considering phylogeny: F3,113 = 98.2, P < 0.001; considering phylogeny: F3,113 = 3.2, P = 0.03), as carnivorous species tend to have larger body sizes than species with other food habits. The large hatching asynchronies of raptors or fish-eating birds is well known (Lack 1954, 1968; Nelson 1978; Newton 1979). It was considered as support for the ‘brood reduction’ hypothesis, because food of raptors or fish-eaters may be especially unpredictable. However, the largest hatching asynchronies were not found in carnivorous species, but in species feeding on plant material (Fig. 4). Little is known about the possible differences in predictability or stability of food sources between different diets, but it has been shown that seeds can be a highly predictable food source (Stoleson & Beissinger 1997b). Intuitively, plant food does not seem to be a less predictable, abundant or regular food source that animal food. Furthermore, we did not find significant differences in hatching asynchrony between species where brood reduction or sibling aggression was reported, and those where they were not reported. Thus, food habits seem to affect hatching asynchrony, but perhaps not through an effect of brood reduction or sibling aggression. Much experimental work testing the brood reduction hypothesis has been done, but, with few exceptions (Magrath 1989; Hébert 1993; see also Wiebe & Bortolotti 1995), most studies rejected this hypothesis (reviews in Amundsen & Slagsvold 1991 and Stoleson & Beissinger 1995; see also Stoleson & Beissinger 1997b).
Plant food is a relatively poor diet and plant-eating birds exhibit slow growth (O’Connor 1984). Thus, high hatching asynchrony in plant-eaters could be considered as support for other hypotheses relating hatching asynchrony to food sources, such as the ‘offspring quality assurance’ hypothesis. This hypothesis suggests hatching asynchrony would assure an optimal growth of first hatched chicks, something especially important when food abundance is poor. It has received support from comparative (Amundsen & Slagsvold 1991) and experimental work (Slagsvold et al. 1995). It is interesting that the lowest hatching asynchronies were found among omnivorous species which may have richer diets.
Hatching asynchrony was also related to social system. Colonial and gregarious species had higher hatching asynchronies than territorial or solitary species. The ‘egg protection’ hypothesis (Bollinger et al. 1990) predicts an early onset of incubation in social species to protect the eggs from intraspecific interference (Beissinger et al. 1998). However, following this hypothesis, we should expect to find an effect of social system on the onset of incubation rather than on hatching asynchrony. The ‘brood parasitism’ hypothesis (Stoleson & Beissinger 1995) also predicts an early onset of incubation and high hatching asynchrony to protect eggs from parasitism. Brood parasitism is more common among semi-colonial and colonial species (MacWhirter 1989; Rohwer & Freeman 1989). It is not clear if this hypothesis predicts an early onset of incubation or a large hatching asynchrony. By having large hatching asynchronies, social birds would assure that parasitic eggs would not be first in the hatching sequence, and that the chicks most likely to reach fledging age would be their own. However, social system may also reflect other constraints on hatching asynchrony not related with parasitism. For example, social species could be more food constrained, given that they live at higher densities.
CONCLUSIONS
Our analysis partly supports the egg viability hypothesis to explain the early onset of incubation. Food habits and social system also seem to play a role in explaining variation in hatching asynchrony. However, our results can not exclude other hypotheses, which may act on a more limited taxonomic scope. For example, we did not find any clear effect of sibling aggression on hatching asynchrony or on the onset of incubation. However, hatching asynchrony could be a mechanism to establish a within-brood size hierarchy that reduces sibling aggression (the ‘sibling rivalry reduction’ hypothesis; Hahn 1981), which has received support from experimental studies in raptors (Forbes 1991; Viñuela 1991; Wiebe & Bortolotti 1994), gulls (Hahn 1981), herons (Fujioka 1985a, b; Mock & Ploger 1987) and boobies (Anderson 1989; Osorno & Drummond 1995).
We conclude that, although several selective pressures could be acting on the onset of incubation and hatching asynchrony, only a limited number seem to act across a large, taxonomically varied group of birds. The observed trend of a balance between the onset of incubation versus hatching asynchrony as clutch size increases suggests that several opposing selective pressures could be acting in a wide array of species. Additional comparative work, perhaps at a finer taxonomic scale, will be necessary to shed light on the main factors affecting hatching asynchrony and the onset of incubation.
ACKNOWLEDGEMENTS
Scott H. Stoleson provided his original bibliographic data base, on which the start of this work was based. Steven R. Beissinger stimulated us to develop this comparative method using phylogenetic methods. Steven R. Beissinger, Juan Moreno and Tore Slagsvold reviewed drafts of this paper. This work was funded by a postdoctoral Fulbright grant at Yale University to Javier Viñuela and by National Science Foundation grant No. IBN-97961565 to Steven R. Beissinger. Completion of the work was funded by DGICYT research project PB94-0070-02-01 and by MEC research contract to Javier Viñuela. The American Ornithologists’ Union and the IOC subsidy committee kindly funded Javier Viñuela to attend the IOC.
REFERENCES
Amundsen, T. & Slagsvold, T. 1991. Hatching asynchrony: facilitating adaptive or maladaptive brood reduction? Acta XX Congressus Internationalis Ornithologici 3: 1707-1719.
Anderson, D.J. 1989. The role of hatching asynchrony in siblicidal brood reduction of two booby species. Behavioral Ecology and Sociobiology 25: 363-368.
Arnold, T.W. 1993. Factors affecting egg viability and incubation time in Prairie dabbling ducks. Canadian Journal of Zoology 71: 1146-1152.
Arnold, T.W., Rohwer, F.C. & Armstrong, T. 1987. Egg viability, nest predation, and the adaptive significance of clutch size in prairie ducks. American Naturalist 130: 643-653.
Beissinger, S.R. 1996. On the limited breeding opportunities hypothesis for avian clutch size. American Naturalist 147: 655-658.
Beissinger, S.R. & Waltman, J.R. 1991. Extraordinary clutch size and hatching asynchrony of a neotropical parrot. Auk 108: 863-871.
Beissinger, S.R., Tygielski, S., Elderd, B. 1998. Social constraints on the onset of incubation in a neotropical parrot: a nestbox addition experiment. Animal Behaviour 55: 21-32.
Bollinger, P.B., Bollinger, E.K. & Malecki, R.A. 1990. Test of three hypothesis of hatching asynchrony in the Common Tern. Auk 107: 696-706.
Cheverud, J. M., Dow, M. M. & Leutenegger, W. 1985. The quantitative assessment of phylogenetic constraints in comparative analyses: sexual dimorphism in body weights among primates. Evolution 39: 1135-1351.
Clark, A.B. & Wilson, D.S. 1981. Avian breeding adaptations: hatching asynchrony, brood reduction, and nest failure. Quarterly Review of Biology 56: 253-277.
Clark, A.B. & Wilson, D.S. 1985. The onset of incubation in birds. American Naturalist 125: 603-611.
Dunning, J.B. Jr. 1996. CRC Handbook of avian body masses. Boca Ratón, Florida, USA; CRC Press.
Felsenstein, J. 1985. Phylogenies and the comparative method. American Naturalist 125: 1-15.
Forbes, L.S. 1991. Hunger and food allocation among nestlings of facultatively siblicidal ospreys. Behavioral Ecology and Sociobiology 29:189-195.
Fujioka, M. 1985a. Sibling competition and siblicide in asynchronously-hatching broods of the Cattle Egret Bubulcus ibis. Animal Behaviour 33: 1228-1242.
Fujioka, M. 1985b. Food delivery and sibling competition in experimentally even-aged broods of the Cattle Egret. Behavioral Ecology and Sociobiology 17: 67-74.
Gittleman, J. L. & Kot, M. 1990. Adaptation: statistics and a null model for estimating phylogenetic effects. Systematic Zoology 39: 227-241.
Gittleman, J. L., Anderson, C. G., Kot, M. & Luh H.-K. 1996. Phylogenetic liability and rates of evolution: a comparison of behavioral, morphological and life history traits. In: Martins, E. P. (ed) Phylogenies and the Comparative Method in Animal Behaviour; Oxford, UK; Oxford Univ. Press: 166-205.
Hahn, D.C. 1981. Asynchronous hatching in the Laughing Gull: cutting losses and reducing rivalry. Animal Behaviour 29: 421-427.
Harvey, P.H. 1996. Phylogenies for ecologists. Journal of Animal Ecology 65: 255-263.
Harvey, P.H. & Pagel, M.D. 1991. The comparative method in evolutionary biology. Oxford, UK; Oxford University Press.
Hébert, P.N. 1993. An experimental study of brood reduction and hatching asynchrony in Yellow Warblers. Condor 95: 362-371.
Hussell, D.J.T. 1972. Factors affecting clutch size in arctic passerines. Ecological Monographs 42: 317-364.
Hussell, D.J.T. 1985. On the adaptive basis for hatching asynchrony: brood reduction, nest failure and asynchronous hatching in Snow Buntings. Ornis Scandinavica 16: 205-212.
Jordano, P. J. 1995. Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant-animal interactions. American Naturalist 145: 163-191
Koenig, W.D. 1982. Ecological and social factors affecting hatchability of eggs. Auk 99: 526-536.
Lack, D. 1954. The natural regulation of animal numbers. Oxford, UK; Clarendon Press.
Lack, D. 1968. Ecological adaptations for breeding in birds. London, UK; Methuen.
MacWhirter, R.B. 1989. On the rarity of intraspecific brood parasitism. Condor 91: 485-492.
Magrath, R.D. 1989. Hatching asynchrony and reproductive success in the Blackbird. Nature 339: 536-538.
Martin, T.E. 1992. Life history traits of open- vs. cavity-nesting birds. Ecology 73: 579-592.
Martin, T.E. 1993. Evolutionary determinants of clutch size in cavity-nesting birds: nest predation or limited breeding opportunities? American Naturalist 142: 937-946.
Martin, T.E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecological Monographs 65: 101-127.
Martins, E. P. & Hansen, T. F. 1996. The statistical analysis of interspecific data: a review and evaluation of phylogenetic comparative methods. In: Martins, E.P. (ed) Phylogenies and the Comparative Method in Animal Behaviour; Oxford, UK; Oxford University Press: 166-205.
Miles, D. B. & Dunhan, A. E. 1993 Historical perspectives in ecology and evolutionary biology: the use of phylogenetic comparative analysis. Annual Review of Ecology and Systematics 24: 587-619.
Mock, D.W. & Ploger, B.J. 1987. Parental manipulation of optimal hatching asynchrony: an experimental study. Animal Behaviour 35: 150-160.
Mooers, A. O. & Cotgreave, P. 1994. Sibley and Ahlquist’s tapestry dusted off. TREE 9: 458-459.
Murray, B. G. Jr. 1985. Evolution of clutch size in tropical species of birds. In: Buckley, P.A., Foster, M.S., Morton, E.S., Ridgely, R.S. & Buckley, F.G. (eds) Neotropical Ornithology, Ornithological Monographs No. 36; Washington D.C., USA; American Ornithologists’ Union: 505-519.
Nelson, J.B. 1978. The Sulidae. Gannets and Boobies. Oxford, UK; Oxford University Press.
Newton, I. 1979. Population Ecology of raptors. London, UK; T&D Poyser.
Nilsson, J.-Å . 1993a. Bisexual incubation facilitates hatching asynchrony. American Naturalist 142: 712-717.
Nilsson, J.-Å . 1993b. Energetic constraints on hatching asynchrony. American Naturalist 141: 158-166.
O'Connor, R.J. 1984. The growth and development of birds. Chichester, UK; Wiley & Sons.
Osorno, J.L. & Drummond, H. 1995. The function of hatching asynchrony in the blue-footed booby. Behavioral Ecology and Sociobiology 37: 265-273.
Purvis, A., Gittleman, J. L. & Luh, H-K. 1994. Truth or consequences: effects of phylogenetic accuracy on two comparative methods. Journal of. Theoretical Biology 167: 293-300.
Ricklefs, R.E. 1965. Brood reduction in the Curve-billed thrasher. Condor 67: 505-510.
Rohwer, F.C. & Freeman, S. 1989. The distribution of conspecific nest parasitism in birds. Canadian Journal of Zoology 67: 239-253.
Sarich, V. M., Schmid, C. W. & Marks, J. 1989. DNA hybridization as a guide to phylogenies: a critical evaluation. Cladistics 5: 3-12.
Sibley, C. G. & Ahlquist, J. E. 1990. Phylogeny and Classification of Birds. A Study in Molecular Evolution. New Haven, Connecticut, USA; Yale University Press.
Slagsvold, T. 1986. Hatching asynchrony: interspecific comparisons of altricial birds. American Naturalist 128: 120-125.
Slagsvold, T., Amundsen, T. & Dale, S. 1995. Costs and benefits of hatching asynchrony in blue tits (Parus caeruleus). Journal of Animal Ecology 64: 563-578.
Stoleson, S.H. 1996. Hatching asynchrony in the Green-rumped Parrotlet: a multiple-hypotheses analysis. PhD Thesis, Yale University, Connecticut, USA.
Stoleson, S.H. 1999. The importance of the early onset of incubation for the maintenance of egg viability. In: Adams, N. & Slotow, R. (eds), Proceedings of the 22nd International Ornithological Congress; Durban.
Stoleson, S.H. & Beissinger, S.R. 1995. Hatching asynchrony and the onset of incubation in birds, revisited: when is the critical period? In: Power, D.M. (ed) Current Ornithology. Vol. 12; New York, USA; Plenum Press: 191-270.
Stoleson, S.H. & Beissinger, S.R. 1997a. Extreme hatching asynchrony, brood reduction, and food limitation in a Neotropical parrot: an experimental study. Ecological Monographs 67: 131-154.
Stoleson, S.H. & Beissinger, S.R. 1997b. Hatching asynchrony in parrots: Boon or bane for sustainable use? In: Clemmons, J.R. & Buchholz, R. (eds) Behavioral approaches to conservation in the wild; Cambridge, UK; Cambridge Univ. Press: 157-180.
Telleria, J.L. and Díaz, M. 1995. Avian nest predation in a large natural gap of the Amazonian rainforest. Journal of Field Ornithology 66: 343-351.
Veiga, J.P. & Viñuela, J. 1993. Hatching asynchrony and hatching success in the house sparrow: evidence for the egg viability hypothesis. Ornis Scandinavica 24: 237-242.
Veiga, J.P. 1992. Hatching asynchrony in the House Sparrow: a test of the egg viability hypothesis. American Naturalist 139: 669-675.
Veiga, J.P. 1993. Does brood heat loss influence seasonal patterns of brood size and hatching asynchrony in the House Sparrow? Ardeola 40: 163-168.
Viñuela, J. 1991. Ecología reproductiva del Milano Negro Mivus migrans en el Parque Nacional de Doñana. PhD Thesis, Universidad Complutense de Madrid, Spain.
Viñuela, J. 1997. Adaptation vs. constraint: intraclutch egg-mass variation in birds. Journal of Animal Ecology 66: 781-792.
Webb, D.R. 1987. Thermal tolerance of avian embryos: a review. Condor 89: 874-898.
Wiebe, K.L. & Bortolotti, G.R. 1994. Energetic efficiency of reproduction: the benefits of asynchronous hatching for American Kestrels. Journal of Animal Ecology 63: 551-560.
Wiebe, K.L. & Bortolotti, G.R. 1995. Food-dependent benefits of hatching asynchrony in American kestrels Falco sparverius. Behavioural Ecology and Sociobiology 36: 49-57.
Table 1. Results of multiple regression analysis using the phylogenetic residuals of variables performed with length of the preincubation period as the dependent variable. The % variance is the percentage of variance in the dependent variable that is explained by the independent variables. Nest type scores increased from open nesters to cavity nesters. Beta is the standardised regression coefficient.

Table 2. Results of multiple regression analysis using the phylogenetic residuals of variables performed with hatching asynchrony as the dependent variable. The % variance is the percentage of variance in dependent variable that is explained by the independent variables. Nest type scores increased from open nesters to cavity nesters. Beta is the standardised regression coefficient.
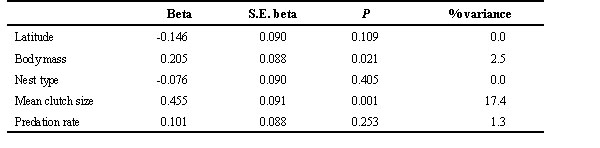
Table 3. Results of multiple regression analysis (using the phylogenetic residuals of variables) performed with mean clutch size as dependent variable. % variance: percentage of variance in dependent variable explained by the independent variables. Nest type scores increase from open nesters to cavity or closed nesters.
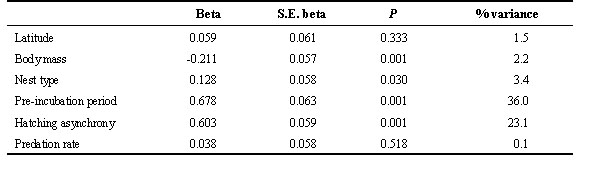
Fig. 1. Results of the phylogenetic autocorrelation model for latitude, body mass, nest type, clutch size, length of the preincubation period, hatching asynchrony, hatching failure and predation rate of eggs and chicks. Phylogenetic autocorrelation coefficient (rho), percentage of variance accounted for by phylogeny (R
2), and significance of phylogenetic effect are also shown.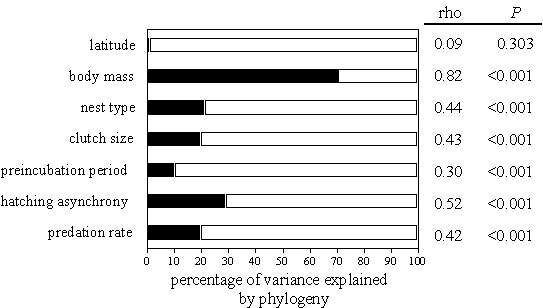
Fig. 2. Distribution of the length of the preincubation period (A), and hatching asynchrony (B) for 117 bird species.
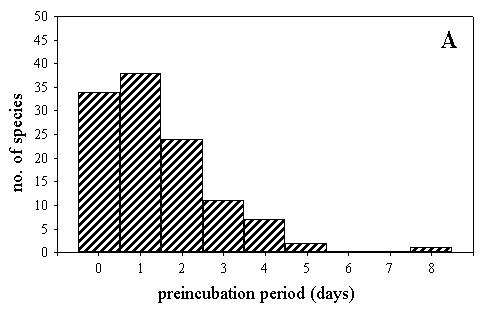
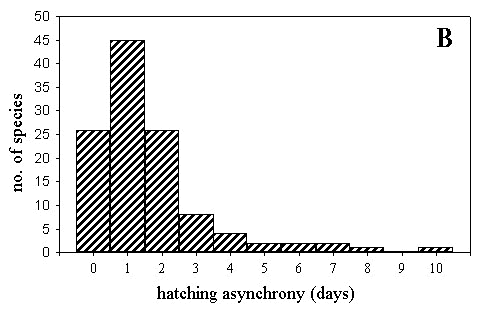
Fig. 3. Variation in hatching asynchrony depending on the food habits of breeding adults. Values presented are phylogenetic residuals removing the effect of clutch size and adult body mass (i.e., adjusted values). Carnivores includes flesh- and fish-eating species. Sample size appears in parentheses above each bar.
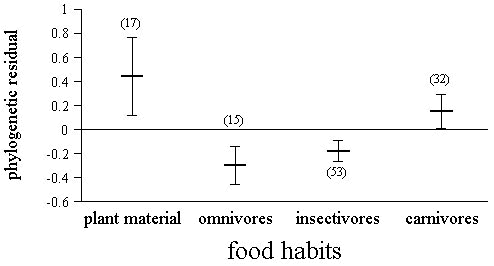
Fig. 4. Variation in hatching asynchrony depending on social system during breeding season. Values presented are phylogenetic residuals removing the effect of clutch size and adult body mass (i.e., adjusted values). Solitary species refers to solitary breeders that are not territorial. Gregarious species are non-colonial social birds. Sample size appears in parentheses above each bar.