S18.3: Impacts of pesticides on reproduction of cavity-nesting birds in apple orchards
Christine A Bishop1, Brian T. Collins2, Pierre Mineau2, Neil M. Burgess3, William F. Read1 & Chris Risley4
1Canadian Wildlife Service, 867 Lakeshore Road, Box 5050. Burlington, Ontario, L7R 4A6, Canada, fax 905 336 6434, e-mail cab.bishop@ec.gc.ca; 2National Wildlife Research Centre, 100 Gamelin Boulevard, Hull, Quebec, K1A 0H3 Canada, e-mail brian.collins@ec.gc.ca; or e-mail pierre.mineau@ec.gc.ca; 3Canadian Wildlife Service, Box 6227, 17 Waterfowl Lane, Sackville, New Brunswick, E4L 1G6, Canada, e-mail neil.burgess@ec.gc.ca; 4510 Gilmour St., Peterborough, Ontario, K9H 2J9, Canada, e-mail crisley@trentu.ca
Bishop, C.A., Collins, B., Mineau, P., Burgess, N.M., Read, W.F. & Risley, C. Impacts of pesticides on cavity-nesting birds in apple orchards. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1043-1057. Johannesburg: BirdLife South Africa.
Apple orchards are frequently sprayed with pesticides however few long term studies of reproduction and studies of the health of birds nesting in orchards have been conducted in the past. Egg and chick survival and pesticide exposure of Tree Swallows Tachycineta bicolor and Eastern Bluebirds Sialia sialis were monitored annually using nest boxes in sprayed and non-sprayed apple orchards in southern Ontario, Canada during 1988-1994. Because many pesticides in current use are not persistent in wildlife tissues, we developed a toxicity score to describe the exposure for each nest and we also measured residual organochlorine pesticides in eggs. The toxicity score was calculated as the product of the extent of the orchard sprayed and the application rate of the chemicals divided by the acute toxicity index of each chemical. We then examined the associations between the reproductive rates and the degree of exposure and toxicity of pesticides applied during the study period and, separately, reproductive rates and the organochlorine residues in eggs. There was a significant increase in unhatched eggs in Eastern Bluebirds as organochlorine concentrations increased in eggs. At the gradient of contamination found in Tree Swallow eggs, there were no trends between reproduction and organochlorine levels. In more than half the study years and over the entire study period, egg fertility and daily survival rates of eggs and chicks of Tree Swallows declined with increased toxicity scores of currently-used pesticides. Reduced egg fertility was detected in Eastern Bluebirds as toxicity scores increased but this only occurred in two years and there was no overall trend for 1988-1994. Daily egg and chick survival were not associated with pesticides in the clutches of Eastern Bluebirds initiated prior to 1st June in each year. The bluebird nests initiated after that date had significantly lower daily chick or egg survival rates concomitant with an increase in pesticide exposure and toxicity in four of seven study years.
INTRODUCTION
Southern Ontario, Canada possesses an unusually temperate climate that provides a unique habitat for wildlife and optimum growing conditions for agricultural crops in Canada. These characteristics promote interactions between a wide diversity of wildlife and agricultural activities in this zone. The area is that part of Ontario located along the Lake Ontario shoreline or south of approximately 43o N latitude (Cadman et al. 1987). It is located in the transition zone between two major forest regions: the Eastern Deciduous Forest, which in Canada is commonly referred to as the Carolinian Zone; and the Great Lakes-St. Lawrence Forest (Cadman et al. 1987). Southern Ontario has warmer than average Canadian temperatures throughout the growing season and there is an ameliorating effect of Lakes Ontario and Erie on the climate which contributes to the existence of a frost-free period of 169 days, and mean annual precipitation of 700 mm which are ideal for tender fruit production (McCuaig and Manning 1982). The floral and faunal assemblage in southern Ontario is diverse and includes many species that are at or near the northern or southern limits of their geographic range (Heagy and McHattie, 1995). Coincidentally these conditions present a valued land resource for agriculture, especially fruit production, due to the mild climate and soils that are mainly class 1 and 2, well-drained and lightly textured (McCuaig and Manning 1982). Some bird species have thrived in the open landscapes typical of agricultural areas while other species persist despite the removal 90% of the forest cover of southern Ontario for agricultural and urban development (Cadman et al. 1987; Kirk et al. 1996). Consequently, wildlife that occur in Ontario are forced to live in wild remnant and fragmented habitats on or near farms and cities or in semi-natural areas such as orchards. Since pesticide use is common in apple orchards in southern Ontario, the purpose of this study was to examine the impacts of these chemicals on the reproduction of songbirds that nest in orchards in southern Ontario.
By 1791, a total of 200 000 inhabitants were established in agricultural settlements along the Niagara Peninsula in southern Ontario (McCuaig and Manning 1982). By the later 1800s, pesticide use on larger orchards was common. ‘Bordeaux’ mixture consisting of 0.3 lbs/10 gallons water each of copper sulphate, and lime was used as a fungicide for apple scab Venturia inaequalis (Beckett, 1913; Emerson et al., 1945). Two arsenic sprays, Paris green (0.33 lbs/ 50 gallons water) and lead arsenate (1.5 lbs/50 gallons water), were used on orchards to control insects, mainly codling moth (Cydia pomonella) (Emerson et al., 1945). Spray applications were made from two to seven times per growing season to control the same pests of concern to apple growers today (Ontario Ministry of Agriculture and Food, 1994). By the 1940s, agricultural publications began describing methods by which these chemicals could be applied efficiently to improve yield and reduce spray costs (Woodworth and Rawlings, 1945). Lead arsenate was still being used but lime-sulphur (0.33 gallon/ 10 gallons water) was beginning to replace Bordeaux mixture because some apple varieties were tarnished by copper sulphate (Emerson et al., 1945).
Around the turn of the century, the use of orchards by birds and the economic value of birds to agriculture and, in particular, to orchards were also being studied. Beginning in the 1880s, ornithologists in North America were noting the regular use of orchards by birds and the value of birds, especially woodpeckers, in the control of codling moth. Downy Picoides pubescens and Hairy Picoides villosus woodpeckers consumed the overwintering codling moth larvae (MacLellan 1958; Kirk et al. 1996). Some birds were also considered pests in orchards and studies on this issue were also common (Kirk et al. 1996). The study of interactions between orchards and bird communities was prominent at the turn of the century but declined in the 1930s and 1940s. This was partly due to the increasing use of pesticides especially the introduction of DDT and its related compounds in the late 1940s which appeared to provide efficient and ideal pest control (Kirk et al 1996). The extensive use of organochlorine compounds continued in orchards through to the 1970s, and the value of birds to agriculture was all but forgotten (Kirk et al. 1996). During this period, the intensity of DDT (30-60 lbs/ acre/year) use in orchards on the Niagara Peninsula was extremely high (Ginsberg and Reed, 1954).
By the 1970s, the scientific focus on birds in agricultural areas became the study of the effects of pesticides on birds. Organochlorine pesticides were causing death of adult birds and other wildlife as well as embryotoxicity and eggshelling thinning in birds (Newton et al. 1986). Most organochlorine pesticides (OCs) were banned from use in North America by 1972, except endosulfan, which remains the only organochlorine compound in use today in orchards in Ontario. In the latter 1970s and 1980s, chemicals that are less persistent than the organochlorine compounds were introduced to control the major pests of apple development. Some of the new compounds such as the organophosphorus and carbamate insecticides are much more acutely toxic than the OCs whereas others such as the synthetic pyrethroids have low acute toxicity to vertebrates but high acute toxicity to insects. Fungicides and acaricides have been developed which have low persistence, and low acute toxicity to vertebrates but are effective in pest control. Despite the introduction of these new chemicals, to this day, bordeaux mixtures are occasionally used and sulphur is commonly used in some orchards to control fungi of apples.
Apple orchards now occupy about 12565 ha of land in Ontario (Statistical Services Unit, 1992). Although there are two commercial organic orchards in Ontario, all other orchards use pesticides to control apple pests. Chemicals are applied on a weekly basis during early April to mid-August in Ontario. Applications are often made as mixtures of insecticides and fungicides.
Birds which nest or feed in apple orchards are frequently exposed to pesticides used in orchards (Blus et al., 1987; Patnode and White, 1991; Fleutsch and Sparling, 1994). Reproductive effects and other sublethal health impacts of pesticides on birds in orchards are of particular concern due to the continual loss of natural habitats which increases the likelihood that birds will utilise semi-natural agricultural areas such as fruit orchards (Graham and Desgranges, 1993). In Ontario, Canada, where this study was performed, organophosphorus (OP) and/or carbamate insecticides are applied up to four times and fungicides are applied bi-weekly during an average apple growing season (OMAFR, 1994). These pesticides are often applied as mixtures of chemicals. There is limited understanding of the effects of pesticide mixtures although additive toxicity is common and some chemical combinations are synergistic in their toxic effects (Thompson 1996). In addition, bird eggshells are permeable to organophosphorus and dithiocarbamate compounds and some are teratogenic through this route of exposure (Hoffman, 1990).
Organophosphorus (OP) and carbamate (CARB) insecticide exposure can depress avian cholinesterase (ChE) levels which can sometimes lead to the death of wild birds in agricultural areas including orchards (Wilson et al 1997; Hooper et al. 1989). In the orchards used in the present study, Burgess et al (in press) had documented cholinesterase depression in both Tree Swallows Tachycineta bicolor and Eastern Bluebirds Sialia sialis that nest in the orchards. The reproductive effects of chemicals used in orchards, especially OPs and CARBs, includes reduced egg production, hatchability of eggs and fledging of young in captive and wild birds (Smith 1987; Patnode and White 1991; Fleutsch and Sparling 1994) yet the mechanism(s) of altered reproductive success remain unclear. Evidence also shows that sublethal exposure to cholinesterase-inhibiting insecticides can change bird behaviour and it has been proposed that this could affect their survival or parenting abilities (Grue, Powell & McChesney 1982; Hart, 1983; White, Mitchell & Hill 1983; Meyers, Cummings & Bennett 1990). Another factor that could affect reproduction in these orchards is food loss since arthropod and other invertebrate food sources for birds are reduced after insecticide spray events (Powell 1984). Reductions in food due to pollution can affect reproductive success in some species of birds (Eeva, Lehikonen & Pohjalainen 1997). Furthermore, the potential effects on reproduction of residual organochlorine chemicals (OCs) have not been integrated into studies examining the impact of in-use pesticides on reproduction despite the high OC concentrations in soils and wildlife in orchards (Johnson et al., 1976; Blus et al., 1987; Hebert et al 1994; Elliott et al., 1994) and the known toxicity of OCs to avian reproduction (Jefferies 1967, 1971; Keith and Mitchell 1993).
Since past studies on the reproduction of birds in orchards have been limited to studies of less than three years, the interpretation of long-term effects and inter-year differences in effects is limited. Therefore, we monitored reproduction in Tree Swallows and Eastern Bluebirds nesting in sprayed and non-sprayed apple orchards in Ontario, Canada during 1988-1994. We determined if there were associations between reduced reproduction and the frequency and degree of chemical exposure to the birds during 1988-1994. This was part of a larger study that also examined behaviour, immune and endocrine function in Tree Swallows nesting in pesticide-sprayed apple orchards.
METHODS
Study Areas and Nest Monitoring
A total of twenty four sites were studied during 1988-1997. Nineteen sites were conventionally managed and sprayed apple orchards, one site was an organic orchard which was sprayed but not with chemical pesticides other than sulphur, and four sites were rural locations which were either pastures where pesticides were never sprayed or orchards that were never sprayed or mowed during our study period. For all but one of the conventionally managed orchards there was at least one year when each orchard was not sprayed. All sites were located in the Great Lakes basin in southern Ontario, Canada (approximate latitude 43o15'N/ longitude 80o2'W).
Eastern Bluebirds and Tree Swallows nest in cavities. To standardise exposure and nesting conditions among study sites, we installed nest boxes which these species readily occupied. In each year of 1988-1994 we monitored nests from mid-April to mid-August.
Associations between organochlorine residues in eggs and reproduction
To evaluate the relationships between organochlorine residues in eggs and reproduction, we collected eggs and analysed them for 19 pesticides and for polychlorinated biphenyls ( Bishop et al., 1996). To remove any confounding effect of in-use pesticides when we evaluated the relationships between reproduction and organochlorine residues, we only analysed the reproductive results of Tree Swallow and of Eastern Bluebird nests which had not been exposed to in-use pesticides during 1988-1994. Also, for both species, the only nests included in the analysis were initiated prior to June 1st in each year. Since eggs were collected in more than one year at a few sites, the average of the pooled concentrations was used in the analysis. A randomisation test (p
£0.05 with sequential Bonferroni test on p values (Rice, 1989)) for trends was used to examine the effect of OC concentration against reproductive success. For clutch size, a one-tailed test for a declining trend with respect to OC level was performed while a one-sided test for an increasing trend was applied to egg fates.Associations between toxicity and frequency of spray events during 1988-1994
Pesticides used in orchards during the study are not bioaccumulative in bird eggs so we lacked actual concentrations of exposure for evaluating the relationship between exposure and reproductive response. We calculated each nest's chemical exposure based on spray application rate, the extent of the orchard sprayed and the relative avian toxicity of each chemical. The experimental unit of exposure was the nest since we assumed fates of all offspring would be correlated.
The toxicity score for each nest was calculated as:
(FACTOR)(RATE) / (INDEX)
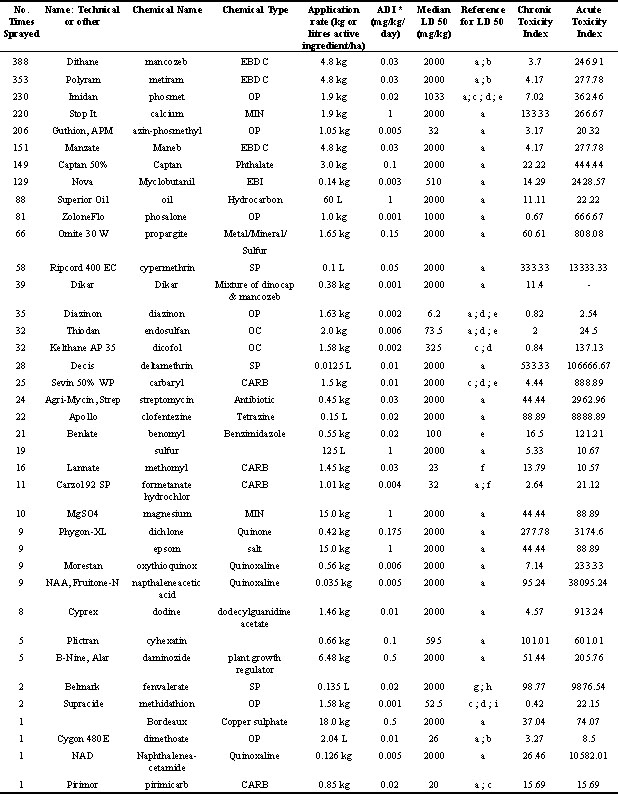
FACTOR was the surface area of the farm sprayed. This was described on the spray schedules received from farmers and ranged from 100% of the orchard to approximately 75%; 50%; 33%; 25%;12.5% of the orchard sprayed; 5% for border spray of the orchard or where only the base of the fruit trees was sprayed and 1% if sprays were only on trees in the row bordering the farm home. RATE was the application rate of each chemical converted to kilograms of active ingredient/ hectare. INDEX was a toxicity index for each chemical. Acute toxicity indices were derived from the amount of contaminated insect necessary to reach the LD 50 for a model adult Tree Swallow (based on typical weight of 20 g; Robertson et al., 1992) or an adult Eastern Bluebird (35 g; Bishop, unpublished data). This ‘lethal meal size’ was based on literature values for LD 50s for a variety of avian species (Table 1). Also, we assumed an average level of contamination of 27.9 ug/g of insect wet weight for an application rate of 1 kg a.i/ha based on a number of literature values for grasshoppers (Stromberg et al., 1984; Leighton and Wobeser, 1987; Forsyth and Westcott, 1994; Hawley and Somers, 1998). The chronic toxicity index was based on the amount of chemical required to reach the allowable daily intake (ADI) for the model tree swallow or model bluebird. Since ADIs have not been developed for birds for all the compounds used in the orchards, the ADIs used were those developed for humans (International Programme on Chemical Safety, 1996).
The daily exposure for each nest was calculated as the summed toxicity scores of all chemicals sprayed per day. The cumulative exposure for each nest was calculated as the sum of all the daily exposures up to and including the last day of exposure being examined in the statistical analysis.
A weighted linear regression was used to examine the relationship between average clutch size and average daily toxicity scores during the pre-incubation period using the number of nests as a weighting factor (Sokal and Rohlf, 1981). The analysis of the relationship between daily toxicity score and proportion of fertile eggs was a logistic regression based on the logarithmic transformation of the total pre-incubation exposure including a term for the background proportion of fertile eggs. The weighted regressions were run using log-transformed proportions of either clutch size or proportion of viable eggs.
For the analysis of associations between toxicity score and reproduction, we designated three phases of reproduction: pre-incubation, incubation and chick-rearing. Since parent birds of both species spend time nest building prior to clutch initiation (Pinkowski et al., 1975; Robertson et al. 1992), they may be exposed to sprays at that time and we designated that as the pre-incubation period. We designated this period as beginning seven days prior to the date of clutch completion and this period varied because clutches varied in size. Incubation continued from the day of clutch completion until the date that the last egg hatched (Pinkowski et al., 1975; Robertson et al. 1992). Chick-rearing continued from the hatching date until all chicks fledged or were found dead in the nest.
Survival probabilities for eggs (Mayfield 1961; 1975) relative to the cumulative toxicity scores during the pre-incubation period and survival probabilities for chicks relative to cumulative toxicity scores during the pre-incubation through the chick-rearing period were assessed by a maximum likelihood estimation significance test. The curve fitting was done using a logarithmic transformation of the toxicity score. Daily survival rates were fitted using logistic regression including a term for background survival rates for each of the eggs and chicks separately. The significance of the effect of exposure was assessed by calculating the difference in the predicted probability of survival from a non-exposed nest and a nest which received the maximum observed exposure. The frequency of times the randomised difference was as extreme as the observed difference was used as the measure of significance after a sequential Bonferroni analysis of the calculated p values was performed. The sequential Bonferroni analysis was based on p=0.05 applied to the number of correlations performed on results for each year, species and time period of nesting. The difference was used because the estimates of the slope and ED 50 could not be used to order the magnitude of the effect of exposure. We ran 1000 randomisations including the observed data as one randomisation (Appendix 1; Sokal and Rohlf, 1981). The analyses were performed using SAS for PC.
RESULTS
Organochlorine residues in eggs
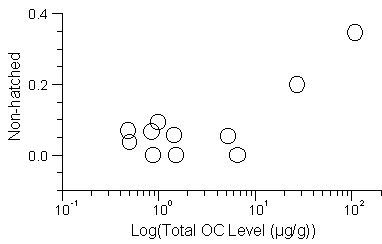
In both species, concentrations of all organochlorine pesticides and PCBs were low (<0.87 wet wt. ug/g) except pptDDE which was detected at much higher levels and showed high variability among sites (Fig. 1). Among sites, the range in pptDDE concentration was 0.43 to 2.56 ug/g in Tree Swallows while range in Eastern Bluebird eggs was 0.37 to 105.1 ug/g. To account for the possible effects of other chemicals or interactions between compounds we analysed associations among reproductive endpoints and organochlorine concentrations in eggs based on the summed concentration of all compounds found in eggs of each species.
For Tree Swallows, there were 10 sites with a total of 200 nests that were not sprayed in 1988-1994. There were no significant trends between increased total OC concentration in eggs and reproductive endpoints. For Eastern Bluebirds, there were 12 sites with nests that were not sprayed during 1988-1994 (N=67 nests). For the combined sample of nests not sprayed during 1988-1994, there were significant associations between increased organochlorine concentrations in eggs and increased occurrence of unhatched eggs (Slope = 0.11; p= 0.008; Fig.1).
Toxicity Indices and Toxicity Scores
The compounds with the lowest toxicity indices and hence the highest toxicity to birds were the organophosphorus and carbamate insecticides. Six of the ten compounds with acute toxicity indices less than 30 mg were OP and CARB compounds (Table 1). Other compounds with low toxicity indices were sulphur, and oil and the organochlorine insecticide endosulfan (Table 1).
Acute and chronic toxicity indices (Table 1) were highly correlated (Spearman R=0.56; p=0.00008). Therefore acute toxicity indices were used in calculations of associations between pesticide toxicity scores and reproduction since more species had been tested and the methods and results of acute toxicity studies were more comparable than those from chronic reproductive studies.
The toxicity scores for nests based on toxicity indices, rate of application and extent of the farm sprayed were similar in most years for tree swallows and eastern bluebird nests initiated before 1st June (early nests). The range in mean cumulative toxicity scores among years was 0.12 to 0.87 for tree swallows. For eastern bluebirds that nested prior to 1st June, the range in mean cumulative toxicity scores among years was 0.42 to 0.71. The bluebird nests initiated after 1st June (late nests) had much higher inter-year variation in toxicity scores than the early nesters. The mean cumulative toxicity scores was 0.83 in 1989 and 2.05 in 1994 with a maximum score of 58.57 occurring on some nests.
Associations between organochlorines in eggs and cumulative toxicity scores
During 1988-1994, there were 388 Tree Swallow nests initiated prior to 1st June on study sites where organochlorine chemicals were measured in eggs. The cumulative toxicity score for pesticides sprayed during 1988-1994 for those nests during pre-incubation through to fledging period varied from 0 to 3.27 while sum total of organochlorine concentrations in eggs varied from 0.74 to 3.50 ug/g. These variables were significantly and positively correlated (Spearman rank R= 0.43; p=0.001; Fig.2).
On farms where organochlorines were measured in Eastern Bluebird eggs, there were 166 nests initiated prior to 1st June. Cumulative toxicity scores at those nests varied from 0 to 4.15 while total organochlorine concentrations in eggs varied from 0.47 to 106.3 ug/g (Spearman rank R= 0.34; p=0.001). This correlation appears to be the result of the large number of observations below 0.10 for cumulative toxicity score and less than 2.0 ug/g organochlorine concentration and hence may be spurious. Nevertheless, these correlations indicate that we cannot completely discount interactions between organochlorine compounds and toxicity of in-use pesticides in Eastern Bluebirds or Tree Swallows.
Associations between Toxicity Scores for In-use pesticides and Reproduction
There were 455 Tree Swallow nests used in the analysis of 1988-1994. Among years sample sizes varied between 18 and 144 nests. The total number of eggs evaluated was 2598. There were no significant trends between declining clutch size and increased daily toxicity scores per nest during pre-incubation (Table 1). For four of seven study years egg fertility declined significantly with increased cumulative toxicity score during pre-incubation (Table 2). Similarly, daily egg survival declined as cumulative toxicity score per nest increased during incubation in 1989, 1991-1993 and the trend during 1988-1994 showed a marginal but not significant decline (Table 2). Daily chick survival also declined significantly with increased cumulative toxicity scores during chick-rearing in three of seven years with marginal declines in two other years (Table 2). The magnitudes of these declines were often small (see Decline (b) Table 2) but for the two daily survival probabilities the cumulative effect over the incubation or chick-rearing periods could be substantial. In some years the decline in fertility was as high as 13% although impacts on egg and chick survival, though statistically significant, were often less than 1% while in some years they were as high as 14% (Table 2).
There was a total of 385 Eastern Bluebirds nests used in the analysis of 1988-1994 reproductive data. Within years sample sizes varied between 5 and 44 nests. The total number of eggs whose fate was evaluated was 930 for birds that initiated nests prior to 1st June (first clutch period) and 796 eggs for nests initiated after 1st June (second clutch period). There was no evidence that clutch size declined with increased daily toxicity scores during pre-incubation. In early nests, there was one year when the proportion of fertile eggs declined significantly with increased cumulative toxicity scores. In late nests (initiated after 1st June) there was only one year showing a significant decline in egg fertility (Table 3).
The daily survival rate of eggs and daily survival rate of chicks in early nests were not significantly associated with current spray exposure (Table 3). However, there were several years with significant trends in the late nesters. Daily egg and daily chick survival decreased significantly with increased toxicity score in three years (Table 3). These declines in survival were significant but not often high. The maximum decline in survival was only 4% (Table 3).
DISCUSSION
Both Tree Swallows and Eastern Bluebirds experienced significant declines in reproduction with increased pesticide exposure and toxicity. In particular, egg survival was affected. Tree Swallows appeared to be more affected than Eastern Bluebirds by pesticides applied during 1988-1994. In Tree Swallows, declines in egg fertility and daily egg and chick survival were associated with increased toxicity scores of pesticides in several years. The early nesting bluebirds were exposed to about the same range of pesticide toxicity scores as Tree Swallows but showed few declines in fertility or survival. In contrast, later nesting bluebirds were exposed to different patterns and degrees of toxicity than the early nesting birds. They experienced significant decreases in egg fertility, egg survival and chick survival particularly in 1994 when toxicity scores for some nests were extremely high. However, it also appears that the high organochlorine concentrations in bluebird eggs in some orchards are as toxic to eggs as pesticides currently in-use.
The results of a behavioural study conducted in 1996 in one of the orchards monitored during 1988-1994 indicated that effects of chemicals in the orchards are likely due to a direct effect on the developing embryo or chick. The use of fungicides and OP insecticides had no effect on incubation periods maintained by Tree Swallow females (Bishop, 1998) which suggests that reduced Tree Swallow egg viability is more likely associated with direct pesticide exposure on the eggs. Possible factors might be direct pesticide contact with the egg via feathers or brood patch of the parent bird or subtle changes induced by OP exposure on the parents' ability to thermoregulate (Rattner et al., 1982) and/or maintain correct egg temperature. In the same study, there were no effects of insecticide use on Tree Swallow chick growth (Bishop, 1998). This also points to mechanisms by which chicks may be affected by spray exposures in orchards. It suggests direct pesticide exposure may be reducing Tree Swallow chick survival through contact with pesticides rather than lack of food resources. Although this is consistent with marginal cholinesterase depression in Tree Swallow nestlings and significant cholinesterase depression in bluebird chicks in these orchards reported by Burgess et al (in press), food reduction by insecticides may be a factor at other locations outside of the sites studied here and should not be completely discounted.
In total, there are now six species of songbirds reported to experience significantly reduced reproductive success associated with the use of pesticides in orchards (Patnode and White, 1991; Fleutsch and Sparling 1994). While most of these species have not demonstrated any overall downward population trends in North America, population sizes of Eastern Bluebirds have been of concern in the recent past. The Eastern Bluebird has been considered a species at risk in Canada (Risley, 1984 ) although populations have increased substantially and current recommendations (Read , 1998) are that the species be downgraded from a listed species in Canada. This recovery is generally attributed to the popularity of bluebird nest box trails and mild winters in recent years (Read, 1998). Many nest boxes are located in rural areas and occasionally within or on the edge of sprayed orchards (McNicholl et al, 1994) therefore the locations of the boxes and potential effects on their eggs should be of concern to trail operators.
The chemicals implicated in reduction of reproductive rates in passerines in other studies in orchards have often been organophosphorus and carbamate insecticides. Our study also indicates that these compounds are potentially highly toxic to birds although we also showed that the organochlorine compounds, in particular DDE are likely to affect egg survival at concentrations found in some orchards despite having been banned several decades ago. Other compounds such as oil and sulphur may also be potentially toxic to exposed birds because of their extremely high rates of application but we caution that the toxicity values used in our indices were limit values and may overestimate the toxicity of these compounds. Although the fungicides used in these apple orchards appear to have low toxicity to birds they are applied repeatedly, also at high rates and often in combination with other chemicals and they do contribute measurably to the cumulative toxicity scores of nests exposed in this study. Since there were no nests that were exposed to OP and CARB alone it is difficult to discern whether their impact is significant alone or in combination with other compounds and this needs to be the focus of future studies. Studies of immune and endocrine function in 16 day-old Tree Swallow nestlings in these orchards show abnormal development in these systems correlated with increasing spray exposure, especially sprays of chemical mixtures (Bishop, 1998). Taken together, studies indicate that birds currently nesting in orchards are lethally and sublethally affected at every critical stage of reproduction and development by past and present pesticide use.
ACKNOWLEDGEMENTS
This study was funded and supported by the Great Lakes Action Plan of Environment Canada, and National Wildlife Research Centre, Canadian Wildlife Service, Environment Canada, Hull, Quebec. We would like to thank Brian Ripley, Pam Leishman and Liz Lissemore of Laboratory Services Division, Pesticide and Contaminants Laboratory, University of Guelph for analysis of endosulfan. We would like to thank Laboratory Services at National Wildlife Research Centre, Hull, Quebec for analysis of organochlorine pesticides and PCBs. We appreciated the long days of field data collection performed by Glenn Barrett, Nancy Mahony and Karen Pettit. We would particularly like to thank Maurice Crickmore, David Hussell, David Inksetter, Gerry Brubacher, Jay Howell, Ed Sarabura, Alex Sanderson, and Cal Wahtras for their understanding and interest in this study. We also thank D. V. (Chip) Weseloh and Donna Stewart for support of this research and reviews of the manuscript.
REFERENCES
Beckett, R.S. 1913. Apple orcharding in Ontario. M.Sc. Thesis. Dept. Horticulture. Ontario Agricultural College: 48 pp.
Bishop, C.A., Ng, P., Norstrom, R.J., Brooks, R.J., & Pettit, K..E. 1996. Temporal and geographic variation of organochlorine residues in eggs of the Common Snapping Turtle (Chelydra serpentina serpentina) and comparisons in PCB patterns in Herring Gulls (Larus argentatus) in the Great Lakes basin in Ontario, Canada (1981-1991). Archiv. Environ. Contam. Toxicol. 31 (4): 512-524.
Bishop, C.A. The effects of pesticide use in apple orchards on health and reproduction of cavity-nesting birds. McMaster University, Hamilton, ON, Canada. Ph.D. thesis. 213 pp.
Blus, L.J., Henny, C.J., Stafford, C.J., & Grove, R.A. 1987. Persistence of DDT metabolites in wildlife from Washington State orchards. Arch. Environ. Toxicol. Contam. 16: 467-476.
Burgess, N.M., Hunt, K., Bishop, C.A., & Weseloh, D.V. 1998. Cholinesterase inhibition in Tree Swallows (Tachycineta bicolor) and Eastern Bluebirds (Sialia sialis) exposed to organophosphate insecticides in Ontario apple orchards. Environ. Toxicol. Chem. 18 (4): in press.
Cadman, M.D., Eagles, P.F.J., Helleiner, F.M. 1987. Atlas of the breeding birds of Ontario. University of Waterloo Press, Waterloo, ON. 617pp.
Emerson, R.A., Howard, R.F., & Westgate, V.V. 1911. Spraying as an essential part of profitable apple orcharding. Bull. Agric. Exper. Station. No. 119. Vol. XXIII. Article IV.
Elliott, J.E., Martin, P.A., Arnold, T.W., & Sinclair, P.H. 1994. Organochlorines and reproductive success of birds in orchard and non-orchard areas of central British Columbia, Canada, 1990-1991. Arch. Environ. Toxicol. Contam. 26: 435-443.
Eeva, T., Lehikonen, E., & Pohjalainen, T. 1997. Pollution-related variation in food supply and breeding success in two hole-nesting passerines. Ecology 78(4): 1120-1131.
Forsyth, D.J. & Westcott, N.D. 1994. Carbofuran residues in grasshoppers and vegetation from aerially sprayed prairie pastures: potential effects on wildlife. Environ. Toxicol. Chem. 13 (2); 299-306.
Fleutsch, K.M. , Sparling D.W. 1994. Avian nesting success and diversity of conventionally and organically managed apple orchard. Environ. Contam. Toxicol. 13: 1651- 1659.
Ginsberg, J.M. and Reed, J.P. 1954. A survey on DDT accumulation in soils in relation to difference in crops. J. Econ. Entomol. 47 (3): 467-474.
Graham, D.J. &. DesGranges, J.L. 1993. Effects of the organophosphate azinphos-methyl on birds of potato fields and apple orchards in Quebec, Canada. Agric., Ecosystems and Environ. 43: 183-199.
Grolleau, F., Caritex, J.L. 1986. Toxicitée par ingestion forcée, de differents pesticides pour la perdrix grise, Perdix perdix L. et la perdrix rouge, Alectoris rufea L. Gibier Faune Sauvage 3: 185-196.
Grue, C.E., Powell, G.V.N., & McChesney, M.J. 1982. Care of Nestlings by Wild Female Starlings exposed to an Organophosphate Pesticide. J. Applied Ecol. 19: 327-335.
Hart, A.1993. Relationships between behaviour and the inhibition of acetylcholinesterase in birds exposed to organophosphorus pesticides. Environ. Toxicol. Chem. 12 (2): 321-336.
Hawley, R.D. & Somers, S.P. 1988. Effects of the exposures of birds to insecticides used for grasshopper control in Alberta. Alberta Environmental Centre Report No. AECV88-R6. 54 pp.
Heagy, A. E. and McHattie, B.J. 1995. Hamilton-Wentworth Natural Areas Summary Report In: Hamilton-Wentworth Natural Areas Inventory ed. Heagy, A.E. 248 pp.
Hebert C.E., Weseloh D.V., Kot L., & Glooschenko V. 1994. Organochlorine contaminants in American kestrels, Eastern Bluebirds and other components of the terrestrial food web in Ontario Canada, 1987-1989. Arch. Environ. Contam. Toxicol. 26: 356-366.
Hoffman, D. 1990. Embyrotoxicity and teratogenicity of environmental contaminants in bird eggs. Review Environ. Contam. Toxicol. 115: 39-89.
Hooper, M.J., Detrich, P.J., Weisskopf, C.P. & Wilson, B.W. 1989. Organophosphorus insecticide exposure in hawks inhabiting orchards during winter dormant-spraying. Bull. Environ. Contam. Toxicol. 42: 651-659.
Henderson, J.D., Yamamota, J.T., Fry, D.M., Seiber, J.N. & Wilson, B.W. 1994. Oral and dermal toxicity of organophosphate pesticides in the domestic pigeon (Columba livia). Bull. Environ. Contam. Toxicol. 52: 633-640.
Hudson, R.H, Haegele, M.A., & Tucker, R.K. 1979. Acute oral and percutaneous toxicity of pesticides to mallards: Correlations with mammalian toxicity data. Toxicol. Appl. Pharmacol. 47 (3): 451- 460.
Hudson, R.H, Tucker, R.K., & Haegle, M.A 1984. Handbook of toxicity of pesticides to Wildlife. Publ. 153, pp. 1-90, U.S. Dept. Interior Fish and Wildl.. Service. International Programme on Chemical Safety 1996. Inventory of the IPCS and other World Health Organisation pesticide evaluations and summary of toxicological evaluations performed by the Joint Meeting on Pesticide Residues (JMPR). United Nations Environment Programme, International Labour Organisation and World Health Organisation. WHO/PCS/97.3. 57 pp.
Jefferies, D.J. 1967. The delay in ovulation produced by pp'DDT and its possible significance in the field. Ibis 109: 266-272.
Jefferies, D.J. 1971. Some sublethal effects of pp'DDT and its metabolite pp'DDE on breeding passerine birds. Overdruk uit: Mededelingen fakulteit Landbouw- Wetenschappen Gent. 36 (1): 34-42.
Johnson, E.V., Guilford, L.M, & Thompson, D.Q. 1976. The effects of orchard pesticide applications on breeding robins. Wilson Bull. 88 (1): 16-35.
Keith, J.O. & Mitchell, C.A. 1993. Effects of DDE and food stress on reproduction and body conditions of ringed turtle doves. Arch. Environ. Contam. Toxicol. 25: 192-203.
Kirk, D.A., Evenden, M.D. & Mineau, P. 1996. Past and current attempts to evaluate the role of birds as predators of insect pests in temperature agriculture In: Current Ornithology. Vol. 13. Chapter 5. Eds. Nolan, V., Jr., Ketterson, E.D. Plenum Press, NY.
Leighton, F.A. & Wobeser, G.A. 1987. Pesticide poisoning in gulls. Can. Vet. J. 28 (3): 108-109.
Lu, F. 1995. A review of the acceptable daily intakes of pesticides assessed by WHO. Reg. Toxicol. Pharmacol. 21: 352-364.
MacLellan, C.R. 1958. Role of woodpeckers in control of the codlin moth in Nova Scotia. Can. Entomol. 90: 18- 22.
Mayfield, H .1961. Nesting success calculated from exposure. Wilson Bull. 73: 255-261.
Mayfield, H. 1975. Suggestions for calculating nest success. Wilson Bull. 87: 456-466.
McCuaig, J.D. & Manning, E.W. 1982. Agricultural land-use change in Canada: process and consequences. Lands Directorate, Environment Canada. Min. Supply and Services, Ottawa, ON. 213 pp.
McNicholl, M.K., Read, W.F., & Weseloh, D.V. 1994. Bluebird nest-box trails in Ontario and their usefulness for bioeffects monitoring of agricultural chemicals. Canadian Wildlife Service Technical Report Series No. 202. Environment Canada, Burlington, ON. 82 pp.
Meyers, S.M., Cummings, J.L., & Bennett, R.S. 1990. Effects of methyl parathion on red-winged blackbird (Agelaius phoeniceus) incubation behaviour and nesting success. Environ. Toxicol. Chem. 9: 807-813.
Mineau, P., Collins, B.T. & Baril, A. 1996. On the use of scaling factors to improve interspecies extrapolation of acute toxicity in birds. Reg. Toxicol. Pharmacol. 24: 24-29.
Newton, I., Bogan, J.A. & Rothery, P. 1986. The role of different organochlorine compounds in the breeding of British sparrowhawks. J. Appl. Ecol. 23: 461-478. Ontario Ministry of Agriculture and Food. 1994. Fruit Production Recommendations. Publication 360. Toronto, ON. 95pp.
Patnode, K.A. & White, D.H. 1991. Effects of pesticides on songbird productivitiy in conjunction with pecan cultivation in southern Georgia: a multiple-exposure experimental design. Environ. Toxicol. Chem. 10: 1479-1486.
Peck, G.K. & James, R.D. 1987a. Tree Swallow in: Breeding birds of Ontario nidiology and distribution Vol 2: passerines. Royal Ontario Museum, Alger Press, Toronto, Canada. 387pp.
Peck G.K. & James R.D. 1987b. Eastern Bluebird in: Breeding birds of Ontario nidiology and distribution Vol 2: passerines. Royal Ontario Museum, Alger Press, Toronto, Canada. 387pp.
Pinkowski, B.C. 1975. Growth and development of Eastern Bluebirds. Bird-banding 46: 273-289.
Powell, G.V.N. 1984. Reproduction by an altricial songbird, the red-winged blackbird, in fields treated with the organophosphate insecticide fenthion. J. Appl. Ecol. 21: 83-95.
Rattner, B.A., Sileo, L. & Scanes, C.G. 1982. Hormonal responses and tolerance to cold of female quail following parathion ingestion. Pestic. Biochem. Physiol. 18: 132-138.
Read, W.F. 1998. History and current status of the eastern bluebird. Bird Trends 6: 23-25.
Rice, W.R. 1989. Analyzing tables of statistical tests. Evolution 43 (1): 223-225.
Risley, C.J. 1984. Status report on the Eastern Bluebird (Sialia sialis). Committee on the Status of Endangered Wildlife in Canada (COSEWIC). 51pp.
Robertson, R.J, Stutchbury, B.J, & Cohen, R.R. 1992. Tree Swallow In: Poole, A., Stettenheim, P. & Gills, F. (eds) Birds of North America 11, Philadelphia. The Academy of Natural Sciences, Washington, DC: The American Ornithologists Union. 27 pp.
Schafer, E.W. Jr., Bowles, W.A. Jr. & Hurlbut, J. 1983. The acute oral toxicity, repellency and hazard potential of 998 chemicals to one or more species of wild and domestic birds. Arch. Environ. Contam. Toxicol. 12: 355-382.
Smith, G.J. 1987. Pesticide use and toxicology in relation to wildlife: organophosphorus and carbamate compounds. USDI Fish and Wildlife Service Resource Publication 170. Washington, D.C. 171 pp.
Sokal, R.R. & Rohlf., FJ. 1981. Biometry. Second edition. W.H. Freeman and Co., New York.
Stromberg, K.L., McEwen, L.C. & Lamont, T. 1984. Organophosphate residues in grasshoppers from sprayed rangelands. Chemistry in Ecology 2: 39-45.Statistical Services Unit. 1992. Agricultural Statistics for Ontario, 1991. Ontario Ministry of Agriculture and Food: Publication 20, 156pp.
White, D.H., Mitchell, C.A. & Hill, E.F. (1983). Parathion alters incubation behaviour in Laughing Gulls. Bull. Environ. Contam. Toxicol. 31: 93-97.
Williams, J.B. 1988. Field metabolism of tree swallows during the breeding season. The Auk 105: 706-714.
Wilson, L.K., Moul, I.E., Langelier, K.M. & Elliott, J.E. 1997. Summary of bird mortalities in British Columbia and Yukon 1963-1994. Tech. Rep. Series No. 249. Canadian Wildlife Service, Pacific and Yukon Region, British Columbia.
Woodworth, H.C. & Rawlings, C.O. 1945. Studies in economics of apple orcharding IV. Spray Management. New Hampshire Agricultural Experiment Station. Station Bulletin 361.
Table 1. Application rates, acceptable daily intakes, LD50s, and chronic and acute toxicity indices for pesticides and other substances sprayed on apple orchards (1988-94).

ADI = Acceptable Daily Intake; EBDC = ethylene-bis-dithiocarbamate fungicide; OP = organophosphorus insecticide; MIN = Mineral; EBI = Ergosterol biosynthesis inhibitor fungicide; SP = Synthetic pyrethroid ; CARB = carbamate insecticide. a = Canadian Wildlife Service 1996. LD 50 pesticide database.Unpublished data; b = Baril et al., 1994; c = Grolleau and Caritez 1986; d = Hudson et al., 1984; e = Schafer et al., 1983; f = Smith 1987; g = Bradbury and Coats 1982; h = Rattner and Franson 1984; i = Henderson et al., 1994; Acute Toxicity Index = amount (mg) of chemical to attain LD50 in a 20 g bird; Chronic Toxicity Index = amount (mg) to reach ADI in a 20 g bird = no LD50 data
Table 2. Results of linear regression and randomization analysis of reproductive results and cumulative toxicity scores for Tree Swallows nesting in orchards and non-sprayed sites in Ontario (1988-1994).
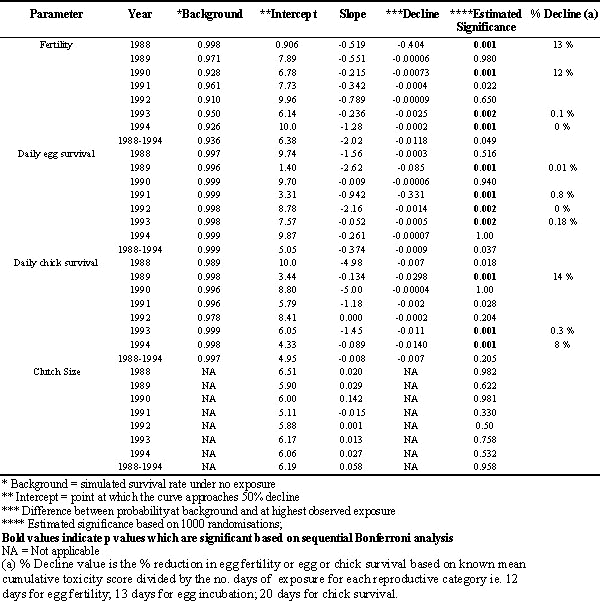
Table 3. Results of linear regression and randomization analysis of reproductive results and cumulative toxicity scores for Eastern Bluebirds in orchards and non-sprayed sites in Ontario (1988-1994).
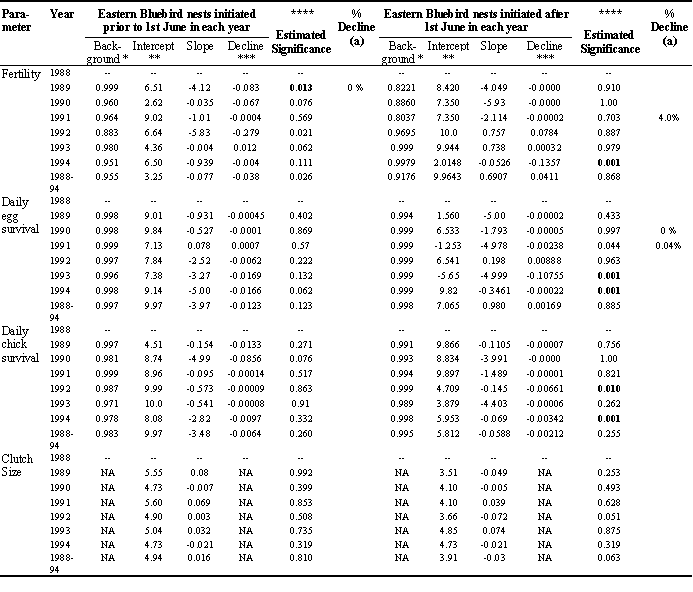
*Background = simulated survival rate under no exposure; ** Intercept = point at which the curve approaches 50% decline; ***Difference between probability at background and at highest observed exposure; ****Estimated significance based on 1000 randomisations; Bold values indicate P values which are significant based on sequential Bonferroni analysis with P< = 0.016; n =5 nests; sample size too small for statistical analysis; (a) = % Decline value is the % reduction in egg fertility or egg or chick survival based on known mean cumulative toxicity score divided by the no. days of exposure for each reproductive category ie. 12 days for egg fertility; 13 days for egg incubation; 20 days for chick survival.
Fig. 1. Total organochlorine concentrations in Eastern Bluebird eggs and incidence of non-hatched eggs in nests not sprayed with chemicals in 1988-1994 and initiated prior to 1 June in each year.