![]()
S25.4: How body water and fuel stores affect long distance flight in migrating birds
Marcel Klaassen1, Anders Kvist2 & Åke Lindström2
1Centre for Limnology, Netherlands Institute of Ecology, PO Box 1299, 3600 BG Maarssen, The Netherlands, fax 31 294 232224, e-mail klaassen@cl.nioo.knaw.nl; 2Department of Animal Ecology, Lund University, Ecology Building, S-22362 Lund, Sweden, e-mail anders.kvist@zooekol.lu.se; 3Department of Animal Ecology, Lund University, Ecology Building, S-22362 Lund, Sweden, e-mail zoo_aake@luecology.ecol.lu.se.Klaassen, M., Kvist, A. & Lindström, Å. 1999. How body water and fuel stores affect long distance flight in migrating birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 1450-1467. Johannesburg: BirdLife South Africa.
Migratory flights by birds are associated with high levels of energy expenditure and high rates of evaporative water loss. Weather conditions are important to migrants as they influence energy expenditure (wind assistance) and water balance (air temperature, pressure and humidity). Flight altitude presumably has little effect on energy expenditure, but has a major impact on water loss as low oxygen partial pressure at high altitude will cause increased ventilation. Although physiologists and ecologists agree on the potential impact of these environmental factors on the migrant’s water and energy budget, only few empirical data are available to allow quantitative predictions. We present updated versions of physiological models predicting maximum flight range imposed by limitations in the migrant’s energy and water balances. Comparing the outcome of these models with studies using birds trained to fly outdoors or in wind tunnels indicate that model predictions appear to be realistic but are associated with considerable uncertainties which can only be reduced by increasing our knowledge on the physiology of bird flight. The models also appear to be valuable in explaining timing of migration in relation to weather conditions and the flight altitude of migrants, although in most cases wind conditions alone explain nearly equally as much of the variation as these highly sophisticated physiological models.
INTRODUCTION
To increase our understanding of life history strategies in birds we often use the rate of energy intake and the economy of its use for life processes as an index of fitness. This is prompted by the fact that all living organisms must obtain and convert energy from their environment and use it for maintenance, growth and reproduction. In many situations this energy is indeed a limiting resource, but there are other potentially limiting factors. For instance, behavioural responses directed at meeting nitrogen requirements rather than energy requirements have been noted in herbivores (e.g., Marken Lichtenbelt 1993), and also in insectivores (Tinbergen 1981). Also water may be an important limiting factor, especially in desert birds (e.g., Schmidt-Nielsen 1964). For trans-Sahara migrants water imbalance was proposed to be a major factor influencing migratory decisions (e.g., Biebach 1990). Biebach (1990) found that many birds rest out in the desert during the day, apparently to take advantage of the less dehydrating conditions for flight prevalent at night. In their pioneering effort to model both water and energy balance in migratory birds, Carmi et al. (1992) showed that, under certain environmental conditions, flight range in trans-Sahara migrants could be limited by dehydration.
The model of Carmi and her colleagues stimulated the study of migratory physiology and behaviour of trans-Sahara, as well as high latitude migrants, in relation to ambient conditions (notably flight altitude). This has led to a further development of the model. Here we give a full account of the model including recent modifications and compare water balance and energy balance predictions of the model with experimental findings. Also, we review model predictions and field empirical data with respect to migratory behaviour (i.e., choice of flight altitude) in relation to meteorological conditions.
THE MODEL
General outline
The model presented here builds on the model of Carmi et al. (1992) and modifications of it by Klaassen (1995) and Kvist et al. (1998). A schematic outline of the model is presented in Fig. 1. In Appendix 1 a toolbox is provided with an executable Pascal program, the source code, a manual, and a series of input data sheets containing program settings used in the simulations presented in this paper.
The model consists of two compartments describing changes in the energy and water budgets during flight. The energy budget compartment calculates the distance travelled relative to the ground and body mass changes due to the catabolism of body stores and metabolic water production, assuming that all energy necessary for flight is derived from body stores. The water budget compartment calculates total water loss and requires additional input variables, such as pre-flight water content of the bird, and ambient humidity and air temperature. The difference between total water loss and inhaled water plus metabolic water production, the last given by the energy budget compartment, yields net water loss.
The changes in water and energy balances result in changes in body mass, which in turn influence flight energetics and the water budget. Therefore, we simulated migratory flight in time steps of 15 minutes, all parameter values being updated after each time step.
The model can run in two modes. In the energy mode (EM) possible adjustments in water balance during flight are neglected and it is assumed that metabolic water influx matches total water efflux, i.e., net water loss is zero. This mode predicts limits to flight range imposed by energy only. In the energy and water mode (EWM), changes in water balance are accounted for and flight range is limited by either water or energy depending on which runs out first during the simulation – energy or water.
The energy compartment
The most important input variables for estimating the energy budget are pre-flight body mass and fuel content, wing span, air pressure and tail-wind vector. The basis of the energy budget is flight cost model 1 of Pennycuick (1989), which calculates the power requirement for flight in relation to flight speed based on aerodynamic theory. When calculating the power at different air speeds, finally leading to an estimate of the air speed and power at which greatest distance can be covered at the lowest cost (i.e. the maximum range speed Vmr, m/s and the maximum range power output Pmr, W, respectively), Pennycuick’s (1989) model accounts for differences in air density and hence changes in ambient temperature, humidity and pressure with flight altitude. However, Pennycuick’s (1989) model does not take wind conditions into account. Therefore, for each air speed (Va, m/s) in Pennycuick’s model, we calculated the wind effect (D Vw, m/s) according to Piersma et al. (1990), which represents the amount of wind-assistance a bird may expect given its migratory direction (md), the wind direction (wd), the wind speed (Vw, m/s) and its own air speed (Va, m/s):
|
(1) |
where ![]() . This wind effect was then added to air speed before
estimating maximum range speed and power output (cf. Pennycuick 1989). This means that in
the calculation of optimal flight speeds, only the energetic consequences of flight speed
are considered and not the consequences for the water budget.
. This wind effect was then added to air speed before
estimating maximum range speed and power output (cf. Pennycuick 1989). This means that in
the calculation of optimal flight speeds, only the energetic consequences of flight speed
are considered and not the consequences for the water budget.
The water compartment
The water balance part of the model is basically identical to the original model written by Carmi et al. (1992), with the exception of a more realistic calculation of respiratory and cutaneous water loss (Eqs. 5 and 12 below) and that the possibility of heat stress is also accounted for. Below we give a full account of the water balance part of the model , since it has never been explicitly published.
The water balance or net water loss (mN, g/s) is determined by metabolic water production (mmet, g/s) and gross water loss (mG, g/s):
|
(2) |
In Eq. 2 mmet is calculated from the maximum range chemical power input (Pmr, W), obtained from Pennycuick’s (1989) model and the composition of the tissue catabolised:
|
(3) |
where fl, fp, fg, and fw are the fractions of lipids, protein, glycogen and water in the catabolised tissue (together adding to 1), hl, hp, and hg and el, ep, and eg are the amount of water (gH2O/gtissue) and energy (J/gtissue) released when lipids, protein, or glycogen are oxidised, respectively (Table 1).
In Eq. 2, mG is the result of respiratory water loss (mr, g/s), cutaneous water loss (mc, g/s) and excretory water loss with faeces (me, g/s):
|
(4) |
We assume that the bird will excrete any surplus water so that gross water loss always is larger than, or equal to, metabolic water production. Thus mN is always equal to or greater than zero. As uricotelic animals can excrete nitrogen without substantial loss of water (Schmidt-Nielsen 1983), for reasons of simplicity we assumed me to be zero in all cases where mmet<mG. It must be noted, however, that me has proven to reach substantial levels in experimental flights of up to 6 h in free-flying pigeons. During these experiments me was estimated at approximately 10% of the total water loss (Giladi 1997).
Some water will be lost through the skin and the lungs. We assume that a bird that cannot balance its water budget will always attempt to minimise water loss. When a bird is heat stressed, however, it tries to dissipate excess heat. Some heat may be stored by a moderate increase in body temperature (Bernstein 1987) but flying birds will try to remain largely homeothermic through regulated evaporative heat loss. Thus, at relatively low operative temperatures, where the flying bird is not heat stressed, it will just try to minimise evaporative water loss. With an increase in temperature the bird no longer exclusively aims at conserving water, but also on remaining in heat balance. The estimation of evaporative water loss (i.e., mr + mc) according to these two objectives requires different calculation methods both of which are presented below. Each calculation method yields an estimate of evaporative water loss. Where both these estimates are identical, the point where thermoregulation through evaporative water loss commences is reached. Always the largest of the two estimates is assumed to represent true evaporative water loss.
Minimum evaporation version for the calculation of mr + mc
Respiratory water loss depends on the rate at which air is inhaled (
VI, L/s at Standard Temperature Ts= 273.15 K, Pressure Ps=101.3 kPa and Dry) and exhaled by the bird (VE, L/s at STPD), and the difference between water vapour density of exhaled air (j E, g/L at STPD) and inhaled air (j I, g/L at STPD):
|
(5) |
|
(6) |
(Note that oxygen extraction is often calculated from measurements of ventilation volume and oxygen consumption corrected to STPD and that several formulae for its calculation are in use. From Eq. 6 it follows that in our case EO2 = VO2 / ( VI FIO2).
Oxygen consumption is calculated from the maximum range power output, obtained from Pennycuick’s (1989) model, the mechanical efficiency (h , assumed 0.23 according to Pennycuick, 1989) and the energy equivalent of oxygen consumption (k , J/L, Table 1).
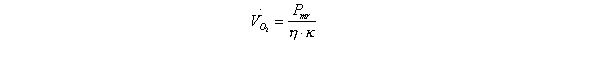 |
(7) |
The bird changes the composition of the inhaled air. Dependent on the ratio between carbon dioxide production and oxygen consumption (the respiratory quotient, RQ, Table 1), this may result in a difference between VI and VE:
|
Note that RQ and k , used in Eqs. 7 and 8, are both functions of the composition of the catabolised tissue (Table 1). If the composition of the catabolised tissue (i.e., the composition of the body stores) in terms of protein, fat and glycogen is known, RQ and k of the substrate mix can be calculated from the RQ and k values of the pure substrates depicted in Table 1.
Water vapour density is a function of air temperature (T, ° C) and relative humidity (RH,%). Based on Murray (1967) we use the following equation to calculate j * (in g/L):
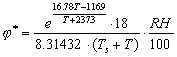 |
(9) |
For the calculation of water vapour density expressed as g/L at STPD we use:
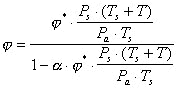 |
(10) |
where Ps is standard pressure (101.3 kPa), Pa is ambient pressure, Ts is standard temperature (273.15 K) and a is the molar volume of gases divided by the atomic mass of water (22.414/18). Using Eq. 9 and 10, j I and j E in Eq. 5 are calculated using ambient temperature (Ta, ° C) and expired air temperature (Texp, ° C) for T, and ambient relative humidity (RHa,%) and 100 for RH, respectively. Texp appears to be dependent on ambient temperature. For flying birds the only published relation as measured in the black duck (Berger et al. 1971) is:
|
(11) |
Carmi et al. (1992) proposed to estimate mc as 10% of mG. Kvist, A., Klaassen, M. and Lindström, Å. (1998) proposed a more realistic calculation of mc based on an allometric relation for the estimation of body surface from body mass (M, g) and estimates of minimal cutaneous evaporation in birds per unit body surface:
|
(12) |
We should again stress that mc is here taken to represent the minimum cutaneous evaporation as we expect it to find in non heat-stressed birds. Note that Eq. 12 assumes mc to be independent of the water vapour pressure gradient over the skin and how this is affected by convection (i.e., flight speed Vmr).
Heat balance version for the calculation of mr+mc
The heat balance of a flying bird is determined by its endogenous heat production and its heat loss through radiation, convection, conduction and evaporation. According to Kvist et al. (1998) mr + mc is a function of Pmr, h , dry conductance under standard conditions in a typical metabolic chamber (Kes, W/° C, which can be approximated by 0.00112
M 0.517; Kvist et al. (1998), Vmr, body temperature (Tb, ° C), operative temperature (Te, ° C, which can be approximated by Ta in the absence of strong radiation; Bakken 1990), and the heat of vaporisation of water (q, which is temperature dependent and amounts to 2400 J/g at 40 °C according to Blaxter 1989).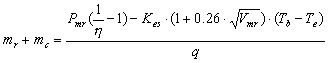 |
(13) |
Carmi et al. 1992, Klaassen 1995, Klaassen 1996, Klaassen, M and Biebach, H. (1998), Liechti F., Klaassen, M. and Bruderer, B. (1999) and Kvist et al. (1998) discuss various aspects of the behaviour of the model described above. Here we present a systematic analysis of the importance of the various model parameters for the outcome of the model. Most of the above mentioned authors used a ‘standard’ Willow Warbler (Phylloscopus trochilus) in their simulations, representing a typical small long-distance migratory passerine. The characteristics of this bird are listed in Table 2 and in Appendix 1 (file MIGRATE.DEF; note that in all of the above mentioned studies slightly different settings were used). Also depicted in Table 2 are the environmental conditions under which we simulated the migration of the standard bird. The maximum flight range of the standard Willow Warbler was estimated as 2760 and 1659 km, using the EM and the EWM, respectively. In all studies that have used earlier versions of the model (Carmi et al. 1992, Klaassen 1995, Klaassen 1996, Izhaki and Maitav 1998, Klaassen, M. and Biebach, H. 1999, Liechti F., Klaassen, M. and Bruderer, B. unpubl.), limits to flight range have been imposed by dehydration rather than by energy shortage, assuming no refuelling possibilities en route for either energy or water. We conducted a sensitivity analysis for the flight range predictions of the model for the standard Willow Warbler. Each of the most important model variables was slightly changed (we arbitrarily used a reduction by 10%), leaving all other variables at their default value and registering the concomitant change in flight range. Using the EM, it came as no surprise that (in order of decreasing effect), the amount of fuel, the energy density of that fuel, the power conversion efficiency, the wing span, and the body drag coefficient were among the most important model parameters. For the more complex EWM many more parameters had a serious effect on maximum flight range. In order of decreasing importance these parameters are expired air temperature (cf. Carmi et al. 1992), fuel composition (i.e., energy density and metabolic water yield, cf. Klaassen 1996), oxygen extraction coefficient (cf. Carmi et al. 1992), and, less surprisingly, maximum water loss, and initial water fraction.
Given the sensitivity of the model outcome for fuel composition it is of note that, although fuel stores were long thought to consist of fat exclusively (Connell et al. 1960; Odum et al. 1964; Odum 1965), carcass analyses have shown that a fraction of the body mass increase of migrants prior to migration consists of protein (McLandress and Raveling 1981; Piersma 1990; Lindström and Piersma 1993, Karasov and Pinshow in press). More recently, energy and nitrogen balance experiments during starvation (Klaassen and Biebach 1994; Klaassen et al. 1997) and 12h wind tunnel flights followed by refuelling (Kvist et al. 1998) supply further evidence that protein is catabolised during migration. Possibly protein content of fuel stores varies among and even within species, but the above-mentioned studies indicate that one should assume fuel stores to consist of a mix of 25% wet protein (i.e., with 77% associated water Blaxter 1989) and 75% fat instead of fat alone. Catabolism of this tissue would thus yield 30.8 kJ/g and 1.02 mlH2O/g (cf. Table 1).
Of critical importance for the model predictions are the flight cost estimates which rely exclusively on aerodynamic theory applied to birds (Pennycuick 1989). With the advent of modern wind tunnels specially designed for the study of bird flight (Pennycuick et al. 1997), scrutiny of this theory has gained momentum and has resulted in findings that stress the importance of applying aerodynamic predictions with great care. Dial et al. (1997) found that the mechanical power ‘curve’ may not at all be U-shaped. Recent work by Pennycuick et al. (1996), Kvist et al. (1998), and Lindström, Å., Hedenström, A. and Kvist, A. (1998) suggest that previously, body drag for birds was overestimated.
The EWM model is very sensitive to changes in expired air temperature. In the current model the calculation of expired air temperature from ambient temperature is based solely on the equation of Berger et al. (1971) from flights of seven Black Ducks, Anas rubripes, that never lasted longer than 18 s. However, recent data from free flying pigeons (G. Michali, pers. comm.) appear to be in line with Berger et al.’s (1971) findings.
Estimates of oxygen extraction efficiency in flying birds are somewhat more numerous (reviewed in Bernstein 1987), but the exceptionally high flight costs in these birds compared with unrestrained birds, as well as the large variation in the estimates of oxygen extraction efficiency also makes this variable an important target for future research. Bernstein (1987) lists oxygen extraction values for nine species of birds in flight ranging from 0.10 to 0.46 (average 0.23). However, we selected the four values for birds in steady state, forward flapping flight which yielded an average of 0.32.
A small decrease in flight altitude (i.e. air pressure) from 2000 to 1800 m above sea level has little effect on flight range using the EWM version of the model (Table 2). However, the range of altitudes birds are found flying almost spans 10 km. If the standard Willow Warbler of Table 2 would choose to fly at sea level instead of 2000 m above sea level, its EWM flight range would increase by 17%, whereas flying at 5000 m would decrease its maximum range by 28%.
In none of the simulated conditions depicted in Table 2 was the bird heat stressed, and therefore we used the minimum evaporation version in our calculation. We found that gross water loss increases rapidly with increasing Te in the EWM model. At an upper critical temperature the bird has to actively increase evaporative water loss to dissipate excess heat. In the model, this upper critical temperature is largely determined by the value assigned to dry conductance. This upper critical temperature for the standard Willow Warbler is estimated to be 32 - 34 °C, almost irrespective of ambient temperature, air pressure and humidity. For the much larger 25 g Thrush Nightingale Luscinia luscinia this upper critical temperature was approximately 29 °C (Kvist et al. 1998). During the migratory period, night time temperatures at low altitude (< 500 m above sea level) over the Negev (Bruderer et al. 1995) and Sahara desert (Klaassen, M. and Biebach, H. 1999) appear to be close to the upper critical temperature of migratory birds, and especially so for large trans-Sahara migrants. Particularly for the daytime situation, with higher ambient temperatures than during the night, the model predictions indicate that migratory birds will have to take risks of heat stress into account when deciding at what altitude to fly.
The model presented here is slightly different from the model of Carmi et al. (1992, with modifications by Klaassen 1995). Notably, the current model takes the possibility of heat stress into account. But also in the calculation of mc and mr in the minimum evaporation version of the water compartment of the model, different outcomes are predicted as a result of improved algorithms and recent findings that have improved parameter estimates.
COMPARING MODEL PREDICTIONS WITH PHYSIOLOGICAL DATA ON FLYING BIRDSAlmost 80 studies estimating the power requirements for flight have been conducted using various techniques (reviewed by Masman and Klaassen 1987, Norberg 1996). In some of these studies body mass loss was used to estimate flight power. However, these studies suffer from uncertainties with respect to body store composition (see above). Wing morphometrics were used in 11 of the remaining studies, allowing the prediction of maximum range and minimum power of flight following Pennycuick, (1989; using a body drag coefficient of 0.08 according to Pennycuick et al. 1996). The data are summarised in Fig. 2. In this figure we present the predicted power range required to fly at air speeds varying from minimum power speed to maximum range speed. We present this range, since a recent compilation of available flight speeds indicated that birds may not always fly at maximum range speed (Pennycuick 1997). Measured power requirements for flight in Fig. 2 appear to be generally higher than predicted power requirements, possibly because in some studies the flights were very short flights and measurements included takeoff and landing (7, 9, 10 in Fig.2) or birds were wearing respiration masks in wind tunnels (8, 12). The studies that yielded estimates close to or below predicted values were studies involving aerial feeders with long pointed wings (1-4, 6) and birds during unrestrained free flights lasting several hours (5, 11). In view of the above data, the use of Pennycuick’s (1989) model seems valid in that it yields reasonable approximations of the ‘true’ power requirements for flight.
To test the results of the EWM, one can measure body mass change before and after a sustained flight. Unfortunately, only four studies exist where body mass loss has been carefully measured during sustained flights lasting at least 1 h and the circumstances under which the birds flew are sufficiently well documented to allow predictions with the presented model. In Fig. 3 the rate of body mass loss in flying Starlings, Sturnus vulgaris, in relation to ambient temperature (Torre-Bueno 1978) is compared with model predictions using various assumptions with respect to power for flight and body store composition. Torre-Bueno assumed 8.9 W for the power requirement for flight (according to measurements by Torre-Bueno and LaRochelle 1978) or approximately twice the value predicted for a bird of this size. This may be due to the fact that the Starlings of Torre-Bueno and LaRochelle (1978) were restrained in a small turbulent test section of a wind tunnel. Torre-Bueno (1978) assumed that Starlings burn fat alone during their flights. We make our predictions using the more realistic composition of 75% fat and 25% wet protein (see above). Furthermore, the Starlings in Torre-Bueno’s (1978) experiments appeared to have body masses close to the species’ lean mass. Therefore, we also calculated the rate of body mass loss assuming flight to be fuelled exclusively by (wet) protein. All predictions of body mass loss, except for the high flight cost - low tissue energy density prediction, are close to the measured values at ambient temperatures below 20 ° C. At higher temperatures all predictions underestimate the actual rate of body mass loss, i.e., the Starlings were heat stressed at lower temperatures than the model predicted.
Another set of data is from flying Pigeons, Columba livia, (Biesel and Nachtigal 1987). In Fig. 4. the rate of body mass loss in the Pigeons in relation to ambient temperature and air speed are compared with model predictions using the same two levels of energy density of body stores as in the Starling example, as well as predicted (Pennycuick 1989) and empirical power requirements of Pigeons flying with respiration masks (Rothe et al. 1987). Only at the lowest ambient temperature of 7.5 ° C did the predicted values appear to be close to the measured rate of body mass loss. At higher temperatures the model predicted the Pigeons to be heat stressed which would result in rapid dehydration. Behavioural observations on the flying birds showed that the Pigeons where conserving heat at 7.5 ° C, trying to dissipate heat at higher temperatures. At 10 ° C their feet came out from under the feathers and upwards of 15 ° C, the birds flew with their bill open (Biesel and Nachtigall, 1987). Given these behaviours it is questionable if not at least part of the heat loss was realised by increased convective cooling. Indeed assuming excess heat production to be dissipated via other routes but evaporative cooling and following the minimum evaporation version in the calculation of body mass loss resulted in a much better fit with the observed data. Adams et al. (1997) measured water turnover in free-flying pigeons at ambient temperatures varying between 18 and 27 ° C using tritiated water. The EWM version of the model predicts their pigeons to be heat stressed but as for Biesel and Nachigall’s (1987) data, Adams et al.’s (1997) data also show a closer fit with the minimum evaporation version of the model. These studies indicate that our understanding of the water budget would profit from a more fundamental understanding of routes of heat loss other but evaporative heat loss.
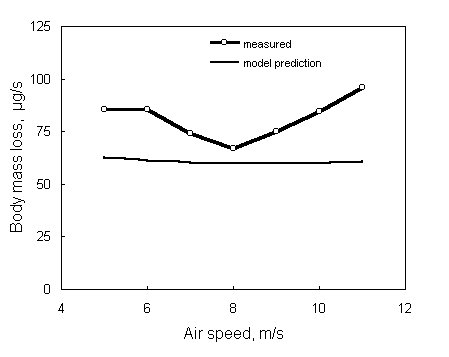
Fig. 5 presents rates of body mass loss in relation to air speed measured in a thrush nightingale (Luscinia luscinia) flying in a wind tunnel (Kvist et al. 1998), along with model predictions using body reserves of 75% fat and 25% wet protein. Measured rates of body mass loss are somewhat higher than model predictions, especially at high and low air speeds. Kvist et al. (1998) suggested that this could be due to a reduction in conversion efficiency at high and low air speeds, or due to heat stress. However, Kvist et al. (1998) did not see any signs of heat stress in the flying bird at any air speed. Furthermore, the model does not predict heat stress to occur, at any air speed, at temperatures below 25 °C, indicating that the conversion efficiency may indeed play a key role in explaining the discrepancies between observations and predictions. However, as previously indicated, many scarcely known parameters determine the model outcome and caution should be taken with respect to its predictions.
COMPARING MODEL PREDICTIONS ON FLIGHT ALTITUDE WITH RADAR DATAKlaassen, M. and Biebach, H. (1999) measured meteorological conditions over the Sahara desert during the autumn migratory period. These weather data served to calculate flight range using the EM and the EWM. Both the EM and EWM predicted optimal flight altitudes in agreement with radar observations carried out during the same period as weather data were collected. Migratory birds flew at an average altitude of 1016 m (above sea level, ASL) during the day and 571 m during the night. The strongly overlapping predictions of the EM and the EWM were due to strong wind assistance at relatively low altitudes. For the spring, with the migratory direction being reversed and tailwinds occurring at high altitude only, Klaassen, M. and Biebach, H. (1999) predicted lower optimal flight altitudes by the EWM compared to the EM. However, Klaassen, M. and Biebach, H. (1999) lacked data to test these predictions. Using a much larger data set, covering both an autumn and spring migratory season, Liechti F., Klaassen, M. and Bruderer, B. (unpubl.) also used altitudinal profiles of meteorological factors to try to explain the altitudinal distributions of nocturnal bird migration recorded by radar above a desert area in southern Israel. For their data the EWM turned out to predict the flight altitudes of migrants slightly better than the EM. Indeed, the differences in predicted flight altitude according to the EM and the EWM were most pronounced in spring, when the EM predicts that migrants should fly high as a result of the prevailing wind conditions (i.e., in the anti-trades above about 1.5 km ASL), but on climb and descent had to cross lower levels, where conditions happen to be better with respect to dehydration problems. However, wind effect alone (Eq. 1) turned out to explain almost the same amount of variance as the EWM. Only in very few cases did wind appear to be a poor predictor of flight altitude and in these cases the EWM proved to be of some value.
So far we have examined small migrants that can swiftly shift between various flight altitudes. For a Willow Warbler with a sustained rate of climb of 5.6 km/h (Hedenström and Alerstam 1992), climbing to the optimal altitude is less time consuming than for a Bewick’s Swan, Cygnus columbianus bewickii, with a rate of climb of 1.5 km/h (Hedenström and Alerstam 1992). Thus if from an energetic viewpoint optimal conditions for flight prevail at high altitude it will take a swan much longer to get there than a small passerine bird. Regular landing from high altitude to drink and compensate for any water imbalance is thus hardly an option for large birds. Indeed, Bewick’s Swans are mostly, though not exclusively, found flying at low altitude seldom exceeding 300 m above ground and appear not to make use of favourable wind conditions in higher air layers (Klaassen, M., Mulder, R. and Nolet, B. A. unpubl.).
FINAL WORD OF CAUTION
The model calculations indicate that migratory flight range may be considerably shortened as a result of a water imbalance. However, in addition to the meagre scientific basis for some of the parameter estimates, birds may also be able to top up their fuel and water en route. Especially water imbalance could often easily be restored, except when crossing oceans and deserts. And clearly, in the latter situation water is most needed and there is none to be had. Nevertheless, a migratory bird performing an ideal migration may at times choose to fly under apparently dehydrating or energy consuming conditions for which it may compensate further en route.
The model also teaches us that water problems may not only occur in warm and dry areas but also when flying at high altitudes, despite low ambient temperatures. The frequency of landing and the time and energy costs involved in the climbs to altitude may then become crucial determinants of flight behaviour (Klaassen, M., Mulder, R. and Nolet, B. A. unpubl.).
ACKNOWLEDGEMENTS
This is publication 2435 of the Centre for Limnology, Netherlands Institute of Ecology. ÅL is supported by grants from the Swedish Natural Science Research Council. We thank Dr. B. Pinshow for his valuable comments on an earlier version of this manuscript.
REFERENCES
Adams N.J., Pinshow, B. & Gannes L.Z. 1997. Water influx and efflux in free-flying pigeons. Journal of Comparative Physiology B 167: 444-450.
Bakken, G.S. 1990. Estimating the effect of wind on avian metabolic rate with standard operative temperature. Auk 107: 587-594.
Berger, M., Hart, J.S. & Roy, O.Z. 1971. Respiratory water and heat loss of the Black Duck during flight at different ambient temperatures. Canadian Journal of Zoology 49: 767-774.
Bernstein, M.H. 1987. Respiration in flying birds. In: Seller, T. J. (ed.) Bird respiration. Vol. 2; Boca Raton: C. R. C. Press: 43-73.
Biebach, H. 1990. Strategies of Trans-Sahara Migrants. In: Gwinner, E. (ed.) Bird Migration, Physiology and Ecophysiology. Berlin; Springer Verlag: 352-367.
Biesel, W. & Nachtigall, W. 1987. Pigeon flight in a wind tunnel. IV. Thermoregulation and water homeostasis. Journal of Comparative Physiology B 157: 117-128.
Blaxter, K.L. 1989. Energy metabolism in animals and man. Cambridge University Press.
Bruderer, B. Underhill, L.G. & Liechti, F. 1995. Altitude choice by night migrants in a desert area predicted by meteorological factors. Ibis 137: 44-55.
Carmi, N., Pinshow, B., Porter, W.P. & Jaeger, J. 1992. Water and energy limitations on flight duration in small migrating birds. Auk 109: 268-276.
Connell, C.E., Odum, E.P. & Kale, H. 1960. Fat-free weights of birds. Auk 77: 1-9.
Dial, K.P., Biewener, A.A., Tobalske, B.W. & Warrick, D.R. 1997. Mechanical power output of bird flight. Nature 390: 67-70.
Flint, E.N. & Nagy, K.A. 1984. Flight energetics of free living Sooty Terns. Auk 101: 288-294.
Gessaman, J.A. & Nagy, K.A. 1988. Energy metabolism: errors in gas-exchange conversion factors. Physiological. Zoology 61: 507-513.
Giladi, I. 1997. Evaporative and excretory water loss in free-flying pigeons - Implications for the study of bird migration. M. Sc. Thesis. Ben-Gurion University of the Negev, Beer-Sheva. Israel.
Hails, C.J. 1979. A comparison of flight energetics in hirundines and other birds. Comparative Biochemistry and Physiology 63A: 581-585.
Hedenström, A. & Alerstam T. 1992. Climbing performance of migrating birds as a basis for estimating limits for fuel-carrying capacity and muscle work. Journal of Experimental Biology 164: 19-38.
Izhaki, I. & Maitav, A. 1998. Blackcaps Sylvia atricapilla stopping over at the desert edge; physiological state and flight-range estimates. Ibis 140: 223-233.
Karasov, W.H. & B. Pinshow. 1998. Changes in lean mass and in organs of nutrient assimilation in a long-distance passerine migrant at a springtime stopover site. Physiological Zoology, in press.
Klaassen, M. 1995. Water and energy limitations on flight range. Auk 112: 260-262.
Klaassen, M. 1996. Metabolic constraints on long-distance migration in birds. Journal of Experimental Biology 199: 57-64.
Klaassen, M. & Biebach, H. 1994. Energetics of fattening and starvation in the long-distance migratory garden warbler, Sylvia borin, during the migratory phase. Journal of Comparative Physiology B 164: 362-371.
Klaassen, M., Lindström, Å. & Zijlstra, R. 1997. Composition of fuel stores and digestive limitations to fuel deposition rate in the long-distance migratory thrush nightingale, Luscinia luscinia. Physiological Zoology 70: 125-133.
Klaassen, M. & Biebach, H. 1999. Flight altitude of trans-Sahara migrants in autumn: a comparison of radar observations with predictions from meteorological conditions and water and energy balance models. Journal of Avian Biology 30: 00-00.
Kvist, A., Klaassen, M. & Lindström, Å. 1998. Energy expenditure in relation to flight speed: what is the power of mass loss rate estimates? Journal of Avian Biology 29:485-498
LeFebvre, E.A. 1964. The use of D2O18 for measuring energy metabolism in Columba livia at rest and in flight. Auk 81: 403-416.
Lindström, Å. & Piersma, T. 1993. Mass changes in migrating birds: the evidence for fat and protein storage re-examined. Ibis 135: 70-78.
Marken Lichtenbelt, W.D. van. 1993. Optimal foraging of a herbivorous lizard, the green iguana in a seasonal environment. Oecologia 95: 246-256.
Masman, D. & Klaassen, M. 1987. Energy expenditure during free flight in trained and free-living Eurasian Kestrels (Falco tinnunculus). Auk 104: 603-616.
McLandress, M.R. & Raveling, D.G. 1981. Changes in diet and body composition of Canada Geese before spring migration. Auk 98: 65-79.
Murray, F.W. 1967. On the computation of saturation vapor pressure. Journal of Applied Meteorology 6: 203-204.
Norberg, U.M. 1996. Energetics of flight. In: Carey, C. (ed) Avian energetics and nutritional ecology; New York; Chapman and Hall: 199-249.
Odum, E.P., Rogers, D.T. & Hicks, D.L. 1964. Homeostasis of the nonfat components of migrating birds. Science 143: 1037-1039.
Odum, E.P. 1965. Adipose tissue in migratory birds. In: Renold, A. E. and Cahill, G. F. Jr. (eds) Handbook of physiology; Washington D. C.; American Physiological Society: 37-43.
Pennycuick, C.J. 1989. Bird flight performance. A practical calculation manual. Oxford University Press.
Pennycuick, C.J. 1997. Actual and ‘optimum’ flight speeds: field data reassessed. Journal of Experimental Biology 200: 2355-2361.
Pennycuick, C.J., Alerstam, T. & Hedenström, A. 1997. A new low-turbulence wind tunnel for bird flight experiments at Lund University, Sweden. Journal of Experimental Biology 200: 1441-1449.
Pennycuick, C.J., Klaassen, M., Kvist, A. & Lindström, Å. 1996. Wingbeat frequency and the body drag anomaly: wind-tunnel observations on a Thrush Nightingale (Luscinia luscinia) and a Teal (Anas crecca). Journal of Experimental Biology 199:2757-2765.
Piersma, T. 1990. Pre-migratory ‘fattening’ usually involves more than the deposition of fat alone. Ringing and Migration 11: 113-115.
Piersma, T., Klaassen, M., Bruggemann, J.H., Blomert, A., Gueye, A., Ntiamoa-Baidu, Y. & van Brederode, N.E. 1990. Seasonal timing of the spring departure of waders from the Banc d’Arguin, Mauretania. Ardea 78: 123-134.
Rothe, H.J., Biesel, W. & Nachtigall, W. 1987. Pigeon flight in a wind tunnel. II. Gas exchange and power requirements. Journal of Comparative Physiology B 157: 99-109.
Schmidt-Nielsen, K. 1964. Desert animals: physiological problems of heat and water. Oxford; Clarendon Press.
Schmidt-Nielsen, K. 1983. Animal Physiology: Adaptation and environment. 3rd ed. Cambridge University Press.
Tatner, P. & Bryant, D.M. 1986. Flight cost of a small passerine measured using doubly labelled water: implications for energetic studies. Auk 103: 169-180.
Tinbergen, J.M. 1981. Foraging decisions in Starlings, Sturnus vulgaris. Ardea 69: 1-68.
Torre-Bueno, J.R. 1978. Evaporative cooling and water balance during flight in birds. Journal of Experimental Biology 75: 231-236.
Torre-Bueno, J.R. & LaRochelle, J. 1978. The metabolic cost of flight in unrestrained birds. Journal of Experimental Biology 75: 223-229.
Turner, A.K. 1982a. Timing of laying by the Swallows (Hirundo rustica) and Sand Martins (Riparia riparia). Journal of Animal Ecology 51: 29-46.
Turner, A.K. 1982b. Optimal foraging by the Swallow (Hirundo rustica): prey size selection. Animal Behaviour 30: 862-872.
Walsberg, G.E. & Wolf, B.O. 1995. Variation in the respiratory quotient of birds and implications for indirect calorimetry using measurements of carbon dioxide production. Journal of Experimental Biology 1: 213-219.
Westerterp, K.R. & Bryant, D.M. 1984. Energetics of free existence in swallows and martins (Hirundinidae) during breeding: a comparative study using doubly labelled water. Oecologia 62: 376-381.
Westerterp, K.R. & Drent, R.H. 1985. Flight energetics of the Starling (Sturnus vulgaris) during the parental period. In: Illyichev, V. D. and Gavrilov, V. M. (eds) Acta XVIII Congressus Internationalis Ornithologici. Moscow: Nauka Press: 392-398.
Table 1. Respiratory quotient (RQ, according to Walsberg and Wolf, 1995), energy equivalent of oxygen consumption (k , according to Gessaman and Nagy, 1988), metabolic water yield (h, according to Schmidt-Nielsen, 1983) and energy yield (e, according to Gessaman and Nagy, 1988) for a uricotelic animal catabolising lipids, glycogen and protein.
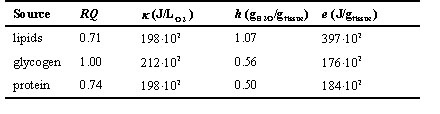
Table 2. Sensitivity analysis of the model for a standard migrating Willow Warbler (see file MIGRATE.DEF in Appendix 1). Each of the most important model variables is reduced by 10%, leaving all other variables at their default value and the effect on maximum flight range using both the energy mode (EM) and the energy and water mode (EWM) model variants is quantified. Using default values for all variables, the maximum flight ranges are 2760 and 1659 km using the EM and EWM, respectively. In none of the simulated conditions was the bird heat stressed (i.e., the minimum evaporation version for the calculation of mr + mc yielded higher values than the heat balance version for the calculation of mr + mc) and the minimum evaporation version was therefore used in the EWM. In all cases where the EWM was used flight range was imposed by limitations of the water balance.
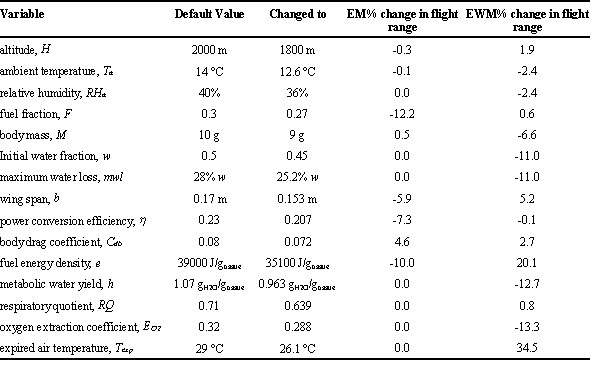
Fig. 1. Scheme of the model used to estimate flight ranges of migratory birds. The model allows the calculation of flight range on the basis of energy stores only, assuming net water loss equals zero (Energy Mode or EM). Alternatively both alterations in energy and water budget are considered simultaneously (Energy and Water Mode or EWM). For detailed explanations see text, Carmi et al. (1992) and Klaassen (1995).
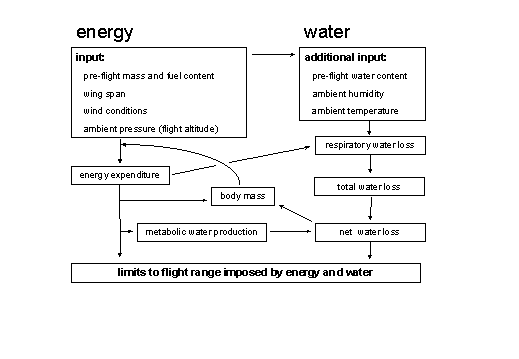
Fig. 2. Empirical estimates of the power for flight and the predicted power for flight according to Pennycuick (1989; using a body drag coefficient of 0.08) The bar representing predicted power estimates are for flight speeds ranging from maximum range speed to minimum power speed. 1: Delichon urbica, Hails 1979; 2: Delichon urbica, Westerterp and Bryant 1984; 3: Hirundo rustica, Hails 1979; 4: Hirundo rustica, Turner 1982a,b; 5: Luscinia luscinia, Kvist et al. (1998); 6: Sterna fuscata, Flint and Nagy 1984; 7: Erithacus rubecula, Tatner and Bryant 1986; 8: Sturnus vulgaris, Torre-Bueno and LaRochelle 1978; 9: Sturnus vulgaris, Westerterp and Drent 1985; 10: Falco tinnunculus, Masman and Klaassen 1987; 11: Columba livia, LeFebvre 1964; 12: Columba livia, Rothe et al. 1987.
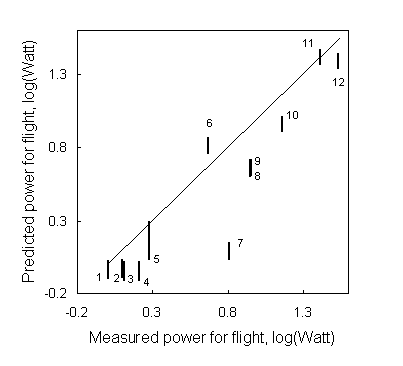
Fig. 3. The rate of body mass loss in flying Starlings, Sturnus vulgaris, in relation to ambient temperature (Torre-Bueno 1978) compared with model predictions using two different body store compositions (i.e., 75% fat and 25% wet protein 30.8 kJ/g and wet protein only 4.2 kJ/g) and two different estimates for the power requirements for flight (the empirical estimate of 8.9 W by Torre-Bueno and LaRochelle 1978 and the predicted value 4.2 W according to Pennycuick 1989). This figure is compiled using STARLING.DEF and STARLING.DAT in Appendix 1.
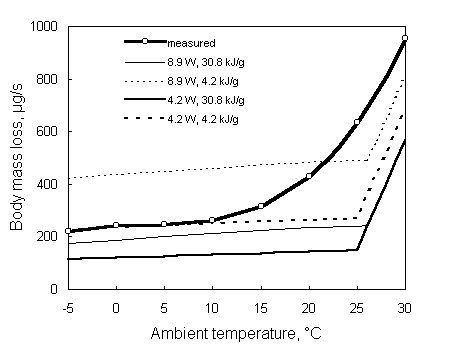
Fig. 4. The rate of body mass loss in flying Pigeons, Columba livia, in relation to ambient temperature (top panel) and air speed (bottom panel; Biesel and Nachtigall 1987) compared with model predictions using two different body store compositions (i.e., 75% fat and 25% wet protein 30.8 kJ/g and wet protein only 4.2 kJ/g) and two different estimates for the power requirements for flight (the empirical estimate of 33 to 39 W, depending on air speed by Rothe et al. 1987 and the predicted values varying between 22 and 25 W, according to Pennycuick 1989). This figure is compiled using PIGEON.DEF and PIGEON.DAT in Appendix 1.
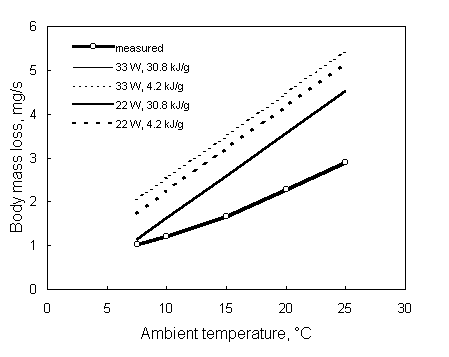

Fig. 5. Body mass loss of a flying thrush nightingale Luscinia luscinia in relation to air speed (Kvist, A., Klaassen, M. and Lindström, Å. 1998). Body stores are assumed to consist of 75% fat and 25% wet protein, resulting in an energy density of 30.8 kJ/g. This figure is compiled using SPROSSER.DEF and SPROSSER.DAT in Appendix 1.
Appendix 1. Toolbox
Copy all files into a single directory under their respective names before using migrat22.
| manual | migrat22.exe | migrat.pas | migrat.def | migrat.dat | starling.def | starling.dat | pigeon.def | pigeon.dat | sprosser.def | sprosser.dat |