S34.2: Foraging patterns of polar penguins
G. Kooyman1, C. Hull2, O. Olsson3, G. Robertson4, J. Croxall5 & L. Davis6
1Scripps Institution of Oceanography, University of California, San Diego, La Jolla, California 92093-0204 USA , fax 619 534 1305, e-mail gkooyman@ucsd.edu; 2University of Tasmania, Hobart 7001, Australia; 3University of Uppsala, Sweden; 4Australian Antarctic Division, Kingston 7050, Tasmania, Australia; 5British Antarctic Survey, Madinghay Road, Cambridge CB3 0ET, UK; 6University of Otago, Dunedin, New Zealand
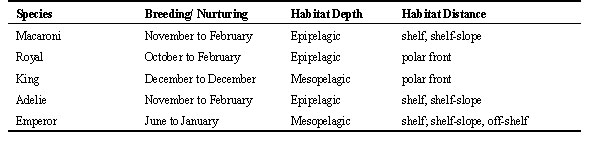
Kooyman, G., Hull, C., Olsson, O., Robertson, G., Croxall, J. & Davis, L. 1999. Foraging patterns of polar penguins. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2021-2039. Johannesburg: BirdLife South Africa.Sub-antarctic and polar penguins have revealed important differences in the distances travelled to foraging areas, the physical and biological characteristics of foraging areas, and foraging patterns. Differences are associated with preferred prey and its abundance. Data were acquired using satellite transmitters and time/depth recorders, the former giving location and rates of travel, the latter diving depths and patterns. Distinctions between travel and feeding dives help to assess foraging success. Data were matched to satellite imagery for determination of sea surface conditions. Sub-antarctic penguins travel further than polar penguins, feed near the Antarctic polar front, and are primarily diurnal feeders. Polar species feed at edges of coastal ice, pack ice, and polynyas. Most locations are neritic. Adelies specialise in krill at shallow depth as do sub-polar Macaroni and Royal Penguins. Emperor Penguins target fish in the mesopelagic zone, which is true also of King Penguins feeding at the Antarctic Polar Front. Hunting with 24h of daylight, polar penguins feed continuously with little hourly variation in depth. Subantarctic penguins show considerable diet differences, with reduced feeding at night.
INTRODUCTION
Penguins are unique among birds in their degree of adaptation to life in a marine environment possessing more complex structural and physiological adaptations for this than any other group of birds. Given these specialisations for life at sea, coupled with potential restrictions on distribution due to flightlessness and the high energy demands of very small marine homeotherms, we might expect penguins to be particularly good as indicators of habitats where prey are concentrated and available for capture.
In this paper, we try to characterise the principal feeding habitats of a range of polar penguin species whose foraging ecology has been the subject of recent study. The data acquired in the last decade on penguin foraging ecology has represented a major breakthrough in the study of marine predators, revolutionising our knowledge of their capabilities and revising our ideas on many aspects of marine science and conservation.
We have selected five of the 17 species of penguins to discuss in detail their foraging habits. All penguins are cold temperate or polar in their distribution, but the five chosen are especially connected to the polar or sub-polar environment. This review is based on some common factors among these five: (1)They are some of the most successful of all the species in terms of abundance and biomass (Woehler 1993); (2)They are also some of the best known because of the detailed studies that have been conducted. For our report this specifically includes the use of satellite transmitters to determine geographic distribution, and time depth recorders to determine hydrographic distribution. (3) At some stage in their life cycle all forage at or near the Antarctic Polar Front (APF), also known as the Polar Front Zone (PFZ). These definitions exclude two species that breed below the APF and commonly, if not exclusively, feed in Antarctic waters. These are the Gentoo, Pygoscelis papua, and Chinstrap, P. antarctica, Penguins. The Gentoo Penguin is not considered because its foraging range is typically restricted to the vicinity of its breeding sites at all times of year (Williams 1991; Wilson et al.1998). In contrast, Chinstrap Penguins may meet the above criteria but no satellite-tracking distribution studies have yet been undertaken. A very recent study using geolocation devices suggests a winter-at-sea distribution adjacent to the marginal ice zone (Wilson et al. 1998).
Three of the species Adelie, P. adeliae, Royal, Eudyptes schlegeli, and Macaroni, E. chrysolophus, Penguins, are surface feeders seldom descending below 50 m to feed mainly on krill. The King, Aptenodyptes patagonicus, and Emperor, A.forsteri, Penguin frequently feed in the mesopelagic region of 200 to 400 m, catching mainly fish.
Distribution, breeding and diet
The congeneric Macaroni and Royal Penguins have very similar breeding biology and ecology (Croxall 1984). The range of the Macaroni Penguin is the sub-Antarctic islands of the Atlantic and Indian Ocean sectors of the Southern Ocean (with the exception of Macquarie Island where the Royal Penguin is endemic), with small outlying populations in South America and on the islands of the Antarctic Peninsula.
Breeding commences in September (Royal) and October/November (Macaroni) with males returning to colonies a week or so before females (Fig.1). After the courtship period, incubation (35 days) and brooding (20-25 days) involves three long (10-20 day) shifts, alternately by males and females. There is bi-parental care during chick rearing with average foraging trips usually in the range 10-48h in Macaroni Penguins. The shortest Royal Penguin foraging trips are for three days during the guard stage. Later in creche the trips lengthen again. After the 55-60 day chick-rearing period, adults feed at sea for 2-4 weeks, returning to the breeding colony to moult (which lasts 20-25 days) before departing to sea for the winter.
Both species take mainly planktonic crustaceans (especially euphausids) and small epipelagic fish. Royals feed primarily on myctophid fish, dominated by Krefftichthys anderssoni, and euphausiids, particularly Euphausia vallentini. Prey items are those associated with the PFZ. Inter-annual differences in diet, degree of digestion of prey and quantity of food brought ashore suggest differences in distribution of prey between years. Diet is consistent across the breeding season in both prey species. About 51% by mass of the diet is euphausiids, and 32% of that mass is made up of E. vallentini. Another 41% by mass consists of myctophids, of which nearly 24% is K. anderssoni (Hindell 1988). Macaroni Penguins tend to specialise in Antarctic krill Euphausia superba at South Georgia (Croxall & Prince 1980; Croxall et al. 1997) but populations in the Indian Ocean have more diverse diets.
King Penguins breed on most sub-Antarctic islands from the Falkland Islands east to Macquarie Island. They are most abundant in the Crozet Archipelago, Indian Ocean sector of the southern ocean.
The King Penguin breeding cycle, which was first described by Stonehouse (Stonehouse, 1956; Stonehouse 1960) at South Georgia, starts in early spring with the moult which takes about a month to complete (Fig. 1). Then courtship and egg-laying take place normally during November and December. The single chick hatches in January/February and is fed until the beginning of the winter, by which time it can be as heavy as an adult. However, the chick remains in the colony through the winter. During the overwinter food shortage chicks fast, but feeding is resumed in the spring for some months until the chick becomes independent. Thereafter the parents moult and soon after may begin a new breeding cycle, although considerably later than in the previous year. Consequently, the King Penguin has one of the most unusual breeding time tables among birds (Olsson 1996). The total time required for these activities exceeds that available during a single summer (Olsson 1996), and the result is that a breeding and moult cycle takes more than one year to complete.
The diet of King Penguins is very consistent. In all areas for which there is information on food habits the main food item by mass are myctophids. In this group the principal species is K. anderssoni. Diet analyses completed at South Georgia, Marion, Crozet, and Macquarie all show the same result (Adams & Klages 1987; Hindell 1988; Cherel et al. 1996; Olsson & North 1997). In short, myctophids are a basic food item of King Penguins, and probably many other predators in the PFZ.
Emperor Penguins are known to breed almost completely around the Antarctic Continent as well as far south into the Weddell and Ross Seas. There are significant gaps in their distribution along the coastline of the Bellingshausen and Amundsen Seas, the east side of the Antarctic Peninsula, and Wilkes Land Coast.
Emperor Penguins begin courtship in the fall (April), and lay in late May to early June (Williams 1995) (Fig. 1). The larger male assumes all incubation duties and about 70 days later, and a few days after hatching, the female returns to relieve the male in late July to mid-August. Over the next five months the female and male come and go to the colony about 10 times to feed the chick. Each trip away tends to get shorter, declining from about three weeks to three days (Kirkwood & Robertson 1997). Around summer solstice the chicks fledge by leaving the colony, and the parents depart to moult (Kooyman et al. 1990). The moult lasts about 35 days.
The primary foods of Emperor Penguins are krill, fish, and squid, which vary according to colony location and season (Cherel & Kooyman 1998; Kirkwood & Robertson 1997; Klages 1989; Putz 1995; Robertson & Newgrain 1996; Robertson et al. 1994).
Adelie Penguins are distributed widely around the whole of the Antarctic continent, including the Antarctic Peninsula and its associated islands north to the South Sandwich Islands and also at the sub-Antarctic island of Bouvetoya (Williams 1995).
Pairing begins in October and laying usually is in November. Incubation lasts about 33 days. Both adults tend the two eggs for shifts lasting 12 days. The guard stage is shared by the parents and after 22 days creches are formed and the parents make foraging trips lasting one to three days. About 55 days after hatching the chicks fledge in mid-February. Moult begins in early march and is complete after about 20 days. Presumably they remain in the pack ice until they return to breed.
Various studies around the continent show that Adelie Penguin diet usually consists of krill, Euphausia crystallorophias, and E.superba (Lishman 1985; Puddicombe & Johnstone 1988; Volkman et al. 1980). In addition to krill some fish, mainly Pleurogramma antarcticum, are taken (Emison 1968; Jablonski et al. 1985).
Foraging
The foraging distribution of the five species is highly seasonal. During chick nurturing in the smaller species there is an intense foraging period that in Adelie and Macaroni occurs close to the colonies, but ranges to the distant PFZ in the Royal Penguin. Similar to Royal Penguins the King Penguin’s primary foraging region is at or near the APF, and it is also up to several hundred kilometres from the colony. In contrast to King and Royal Penguins, the Emperor Penguin, with a similarly long period away from the colony remains much closer during both the summer and winter. The foraging ranges are from about 100 to 200 km from the colony.
METHODS
Satellite technology
The introduction of technologies that have made possible the use of the NOAA satellites ranges from imagery of sea ice, storm patterns, sea surface temperatures, and primary productivity concentrations to detailed tracking and recovery of diving records. The new knowledge about the vastness of the ocean environment that some species utilise is awesome, both in their remarkable travel and habitat needs as it is intimidating to consider appropriate conservation measures needed to protect them. The treaties that will be needed between countries, and the task of enforcement rival any land or ocean use agreements ever before ratified.
This revolution began with the application of time depth recorders (TDR) to marine mammals, but in a few years the miniaturisation had reduced the mass of the recorders from about 500 g to 50 g. At this level the TDR’s can be applied to birds without serious consequences.
The matter of size is still a major problem for satellite transmitters. The mass of one of the smallest units is about 80 g, and the largest is about 230 g. Linear dimensions are now about 9.5 x 4.2 x 2.0 cm.
RESULTS
Sub Antarctic penguins
Macaroni Penguin
The most detailed diving and foraging data come from the chick-rearing period (Croxall et al. 1993; Trathan et al. 1998; Boyd & Croxall unpublished data). Time-budget data (from TDRs and at sea observations both indicate that at this time most birds are feeding about 40-50 km from their colonies over the outer shelf and, in smaller numbers, at the shelf break some 60-70 km distant (Trathan et al. 1998). Diving is assiduous, with very long bouts of continuous activity. Daytime depths average 20-25 m (mean 29m) and night time dives less than 6m (maximum 11m). Even maximum dive depths (to 115m) only reach mid-water depths over the shelf. The summer diet is almost exclusively Antarctic krill, E. superba, averaging about 98% by mass over 12 years of study (Croxall et al. 1997) but often with small numbers of tiny (<2cm long) juvenile notothenioid fish. Other fish species, including lantern fish K. anderssoni, are rare in the diet. The at-sea distribution of South Georgia Macaroni Penguins at other times of year is poorly known. During incubation, foraging trips to the APF would be well within range and some aggregations of birds have been observed there. Winter range is presently entirely unknown, except that birds are not observed in shelf waters around South Georgia at this time.
Royal Penguin
All foraging activity occurs offshore, to the southeast of the Sandy Bay colony of Macquarie Island (54o 33' 51' S, 158o 54' 11' E), and south of the Campbell Plateau over the Emerald Basin in water of 2 to 6o C (Fig . 2). During incubation Royal males from Sandy Bay travel up to a maximum distance of 660 km, while females may travel as far as 415 km (Hull et al 1997). These trips take an average of 22 days for the males and 14 days for the females. During the guard stage the distances decline to 116 km, and then increase to 201 km when the chicks are in creche. Diving is predominantly during daylight hours spending most of the time at depths of less than 60 m. The water in this region is 4000 - 5000 m deep. There is a significant relationship between the duration of the foraging trip and the maximum distance travelled (r2 = 0.475, F1 = 6.336, P < 0.04). The degree of overlap of foraging zones for different stages in reproduction are low (average 22.4%), indicating discrete foraging zones across the breeding season (Hull 1997; Hull et al. 1997).
King Penguin
From January to mid-April, during the brooding stage, King Penguins from Possession Island colonies at 47oS, Crozet Archipelago, range from 144 to 689 km from the colony (Fig. 3a). The trips last from 7.9 to 32.4 days, the average is 16.8 days, and the average speed is 40 to 100 km d-1 (Jouventin et al. 1994; Guinet et al. 1997) (Fig. 3a). Most of these journeys are to the PFZ between 45 and 50o S., where the water temperatures are from 4 to 5oC. En route to this area the birds travel at an average speed of 70 km d-1. While in the area they move around at 54 km d-1, and the return trip is direct at 74 km d-1. Using satellite-based information on surface sea temperatures, Guinet et al. (1997) noted that the preferred temperature for hunting birds is 4 to 5oC. They made the cogent argument that birds from more southerly colonies that are below the PFZ, such as South Georgia and Heard Island, would swim north to the PFZ. That is indeed what they do at South Georgia (Fig. 3b). Working from Husvik, South Georgia, in 1994 Olsson (unpub observ) recorded the travels of four birds during March. All of the penguins swam north to 50 to 53oS, in the region of the PFZ (Fig. 2b). The cycles lasted from 11 to 30 days. Although these are similar to the trip durations of the Crozet birds studied in 1992 and 1993, the South Georgia birds were experiencing a year of low food supply where there was nearly 100% loss of chicks. The cycles were also long compared to previous years when many trips were only two days (Olsson, unpub observ).
While on these trips the diving depth of King Penguins ranges from 50 to 300 m (Bost et al. 1997; Kooyman et al. 1992a; Putz & Bost, 1994; Olsson, unpub observ). Diving almost continuously while at sea the birds’ depth range tracks the daily light cycle closely. At night the diving activities are usually less than 50 m and through the transition from night to day the depths gradually increase into a depth range of usually 100 to 200 m (Fig. 4) (Kooyman et al. 1992). Originally it had been interpreted that the main hunting period was at night on shallow dwelling prey (Kooyman et al.1992). Using stomach temperature sensors it has been shown that the main feeding time is not at night when a vertical migrating prey would be at shallow depths, but rather during the day when they are deep (Bost et al. 1997; Handrich et al. 1997; Putz & Bost 1994). At this time lanternfish are less active and possibly more concentrated so that capture rates would be higher, and the effort more productive despite the deep diving and long duration of the dives. However, some caution should be considered in this interpretation. Sensitivity of the stomach recording device may be inadequate for measuring prey during short dives, lower capture rates per dive, and possibly higher digestion rates because of the lack of bulk feeding and stomach cooling. In addition, the cardiovascular responses to diving may be quite different between deep and shallow dives. Instead of restricted blood flow to the stomach, blood flow may be maintained in the short, shallow dives (Kooyman 1989; Kooyman et al. 1992b). This would not only help maintain stomach temperature, but also enhance digestion even while diving. Consequently, the effect of one or two fish of a two or three gram body mass would have little influence on stomach temperature or the stomach probe.
Antarctic penguins
Adelie Penguin
Foraging behaviour of Adelie Penguins has been studied at several different sites and with varying results. From work in the Antarctic Peninsula the birds range about 20 to 50 km from the colony while raising chicks (Fraser & Trivelpiece 1996). In the Ross Sea the birds may range widely and apparently randomly and up to 272 km from the colony during the incubation period (Davis & Miller 1992). However, during the guard stage most foraging ranges are within 15 km (Sadleir & Lay1990). Long trips were also recorded for birds from Bechervaise Is., Mawson Coast, where the maximum distance during the incubation cycle was 341 km, but more normally was about 150 km (Kerry et al. 1996) (Fig. 5). During the chick brooding period the distances ranged from two to 135 km as the birds travelled to the shelf break. In both of these studies some caution in the results is warranted because the cycles were much longer than controls. In a study with only VHF transmitter tracking at Hukuro Cove (69oS, 39o35'E), Enderby Land, birds only departed for from 5 to 20 h, and fed near the colony in a wide variety of conditions from tidal cracks, and ice holes to the ice edge (Watanuki et al. 1993).
Diving depths while hunting range from about 3 to 98 m with and average of 26 m (Chappell et al. 1993). Similarly shallow dives averaging from 7 to 12 m occurred over three seasons of study (Watanuki et al. 1993). In this study the diet ranged from nearly 100 % krill by mass in January to as little as 1.5 % krill in late January and early February. At this time fish, especially Antarctic Silverfish, dominated the diet.
Emperor Penguin
Data for winter females exists only for Mawson coast colonies and for two years (1993 and 1994) only (Kirkwood & Robertson 1997a; Wienecke & Robertson 1997). In the winter of 1994, 12 females departing Auster colony (67 23S; 64 04 E, Mawson coast) equipped with satellite transmitters and dive recorders took 8 ± 3 days to cross about 70 km fast-ice to open water, their zig-zagging route averaging 175 km travelled, or about 22 km/day (Fig. 6a). Females took 5 ± 2 days to return over a similar distance at the completion of their winter foraging. Trip lengths of winter females averaged 76 (± 9) days and about 17% of this time was spent traversing fast ice.
The most comprehensive information on males exists for colonies in the Ross Sea (Ancel et al., 1992), near Dumont D' Urville (Ancel et al. 1992) and Mawson coast (Kirkwood & Robertson 1997b; Wienecke & Robertson 1997). Male Emperors departing colonies at hatching in late July travelled 12-13 km/ day across the fast-ice each way. Trip durations of fast-breaking males averaged 18 (± 4) days and varied seasonally depending on extent of the fast-ice and foraging successes. About 32% of male trip lengths were spent commuting between colony and open water. During August and early September chick brooding trip durations averaged 11-18 days for females and males respectively, and shortened as the breeding season progressed.
Ten winter females tracked from Auster in 1994 stayed within close proximity of the colony in their 76 days away. Average foraging radii was 133 ± 27 km, maximum distances from Auster ranged from 91 - 255 km and travel speeds averaged 17 ± 6 km/d. Although the total foraging area for the 76 day trip was 11,419 km-2, 50% of foraging days were spent in only 15% (1,700 km-2) of this area (Fig. 6a). The foraging area occurred in pack-ice covered waters 500-1000 m deep over the continental shelf and shelf break. The foraging area included a polynya between the outer limit of the fast-ice and the southern limit of the pack-ice. In both 1993 and 1994 the breeding males also foraged in this area.
Daily foraging durations of females in June and July were limited by the short day lengths of winter. Females spent only 4.8 ± 0.1 hours per day diving, entering the water at 0930 ± 1.35 h and exiting the water at 1430 ± 0.97 h (n = 14 penguins; 499 days). Females foraged on 93.2 ± 7% of their days at sea and had rest days on the remainder. The commonest depth strata frequented by females were 10-50 m and 100-200 m. In the limited day length of winter females were able to perform only 25.9 ± 7.7 dives/day.
Time spent swimming increased as day length increased. In August the males were in the water for 7.83 ± 1.5 hours/day. In September both females and males were in the water 12.23 ± 1.25 hours/day. Accordingly, the penguins' dive rates increased from 92.7 ± 28.5 dives/day in August to 149 dives/day (both sexes combined) in September. The commonest depth strata frequented by both sexes in August and September were 20-200 m.
Birds from the Coulman Is travel a maximum distance of about 100 to 200 km from the colony during foraging (Fig. 6b). In November of 1993, towards the end of the nurturing cycle, parents from the Coulman Island colony in the Ross Sea made trips of an average length of 14.9 days, averaging 213 dives day-1 (Kooyman & Kooyman 1995). About 26% of foraging dives were between 21 to 40 m. However, 2.6 % of the dives were greater than 400 m. Dives occurred throughout the 24 h of the day, but dives greater than 400 m occurred only between 0700 and 2000 local time.
DISCUSSION
Almost all data are the result of summer studies, except for one track of a King Penguin (Jouventin et al. 1994), and the winter studies of Emperors. During the summer months Macaroni and Adelie Penguins are able to generally feed near shore, whereas the Royal Penguin is for some reason required to journey, at some stages, considerable distances. Why there is such a difference between Macaronis at South Georgia and Royals at Macquarie is unknown. The Royals seem to be dependent on prey at the APF where there is a convergence of different water masses. Presumably the main difference in feeding habitat reflects the relative size of shelf (Macquarie very small) and the abundance of shoaling zooplankton around South Georgia. However, it is not impossible that South Georgia birds reach the APF at some times of year, though they may be able to subsist on shelf resources throughout the summer in most years.
Adelies and Macaronis are more associated with the water characteristics of the neritic zone near the colonies. In addition, Adelies have a close association with pack ice, and it is postulated that this is one of the distinguishing characteristics of their foraging habits compared to the Chinstrap Penguin which prefers more open water (Trivelpiece et al. 1987).
Both Macaroni and Adelie Penguins rely substantially on euphausiids, but Royal Penguin diet is more dependent on fish. Curiously, the myctophid fish is the same species as that of the King Penguin, yet they dive much less deeply, and mainly in the daytime. Do they catch the fish mainly at night while it is near the surface, or is there enough of a broad distribution of myctophids for royal penguins to pursue them profitably in the daytime?
In summer and winter king penguins major diet items are myctophids. Their hunting grounds are the mesopelagic zone of the antarctic polar front. There are no data for King Penguin foraging ranges at Macquarie Island, but it is likely that the ranges are similar to that of king penguins at South Georgia and Crozet, and that they travel to the APF. If so, then they may overlap with royal penguins, and be in competition for the same myctophid prey. This is in contrast to the association of kings and macaronis at South Georgia, in which there is a clear separation in foraging range, depth and prey.
Sub-Antarctic species, whether they feed far from the colony or nearby, there is no overlap with other colonies except possibly those metapopulations occurring within the same island group. This is simply because the islands are too widely spaced for any overlap in foraging during the breeding season (Fig. 7). However, Macaronis have very substantial overlap with Antarctic Fur Seal in terms of diet, diving depth and foraging area. Indeed, the massive increase in the latter may account for the progressive population reduction in the former. Also, Macaroni Penguin populations tend to be much larger in relation to available foraging areas (e.g. the northwest South Georgia population of Macaronis equals perhaps twice the world population of Adelies.
In contrast to the great distances between sub-Antarctic islands, Adelie colonies are large and closely spaced in many areas of the Antarctic (Woehler 1993). In the western Ross Sea there are about 20 colonies along the Victoria Land Coast. Even though the foraging range from each colony may not be great, they must be overlapping with other nearby colonies. It would seem that of the species discussed they and Macaroni Penguins may be the most susceptible to resource depletion near the colonies during the breeding season. This would be especially so, because some of the colonies are so large and the birds forage nearby. Recently krill consumption by species with overlapping foraging ranges was estimated (Croll & Tershy, 1998). However, details about the source and size of the prey population is vague. More refined information is needed before colony increases or declines can be attributed to competitive interaction for resources.
Emperor penguins have little competition with any other species of bird. They hunt much deeper than Adelie Penguins, and in those areas such as the Weddell Sea and Mawson Coast where they feed on krill, most of the krill predation occurs before Adelie Penguins return in the spring. There is overlap in foraging areas of at least two Emperor colonies in the Ross Sea, and possibly this is so between other colonies around the continent considering their proximity to each other (Fig. 7). Perhaps one of the most frequent overlaps in area, depth and prey is that of Emperor Penguins and Weddell Seals in the Ross Sea. Here they both feed on the Antarctic Silverfish, dive to similar depths for the prey, and feed in the areas throughout the year. A major separation is that many if not most Weddell Seals remain within fast ice, while Emperor Penguins hunt offshore.
Except for Emperor Penguins there is little known about winter movements or foraging behaviour of polar penguins. Emperors are remarkably conservative in their travel from the colonies. Even the females that have two months to disperse appear to travel only 100 to 200 km from the colony (Kirkwood & Robertson 1997a; Wienecke & Robertson 1997). Instead it is the juveniles that range widely, reaching the APF where if they lingered into winter they might overlap with King and Royal Penguins (Fig. 7). Most likely they do not, but rather move south to meet the expanding pack ice (Robertson, pers observ). While adults committed to breeding demands probably remain restricted to more local travels the juveniles from the various colonies ringing the continent may mix. Even to a much greater degree would be the case of all Adelies, adult and immature, as they travel toward the pack ice edge in winter (Davis et al. 1996).
This is less likely for the sub-Antarctic penguins. Their island colonies are so far separated that only the most dedicated wanderers are likely to meet individuals from other islands, or to reach other islands themselves. This is appreciated from the shortness of the tracks, as notable as they are, relative to the vast distances of the southern ocean. Such distances suggest that although the birds may deplete some local areas, the resource is probably drawn from an almost unlimited reservoir that might quickly replenish the depletion given a small amount of release from predation.
In summary, two of the three sub-Antarctic penguin species breed and complete the chick rearing in the summer (Table 1). The Macaroni Penguin from South Georgia Island conducts this cycle through the months of November to February, foraging in the epipelagic zone close to the colonies. Their travels after moult until the next breeding cycle are unknown. Royal Penguins from Macquarie Island begin breeding in October and fledge the chick by February. Travel during foraging is extensive as they feed in the epipelagic zone at the APF. Similarly, King Penguins that breed and fledge a chick over a period of one year from December to December journey far to the APF where they pursue prey in the mesopelagic depths, while catching mostly fish of the same species as the Royal Penguin. After the moult and through the winter the chick is left to fast while the parents range further along and perhaps south of the APF. Adelie Penguins arrive to reproduce along the Antarctic Continent in November and complete the process in February. Usually they remain near shore and near the colony, but some will travel over 300 km from the colony. Their food is mainly euphausiids captured at depths of less than 30 m. After the moult the birds winter along the pack ice edge a short distance from the continent at a latitude of about 65 to 66o S. Birds travelling from high latitude colonies of the Ross Sea must travel a considerable distance to reach this region (Davis et al. 1996). Emperor Penguins initiate breeding in the fall when other penguins are involved only in their individual needs. However, this early start enables Emperor Penguins to fledge their chick by mid-December, sooner than any other antarctic penguin. While the juvenile Emperor Penguins travel to the APF the adults moult in heavy pack ice sometimes a considerable distant from the colony. Their distribution for the two months after moult, before they return to the colony is unknown. Finally, around the sub-Antarctic islands the oceans are so vast that the birds might disperse with few land barriers, and possibly little intra or interspecific competition because, even though there may be much overlap in the prey sought, they are geographically separated. Around the Antarctic Continent the Emperor and Adelie Penguins may overlap in range, but this is probably seldom inter-species competition for prey or the depth to which they seek their food. On the other hand, intra-species competition may occur often as these two species coalesce into large groups during the winter (Kooyman, pers observations). However, in most cases the benefit of flocking may outweigh the disadvantages.
ACKNOWLEDGEMENTS
The writing of this paper was supported by NSF grants OPP96-15390 and NSF OPP98-09161 to G.L.Kooyman. Winter impressions of the aggregations of Emperor and Adelie Penguins were obtained while cruising the Ross Sea on the RVIB N.B.Palmer during May and June of 1998. Robert Vandam commented on an early draft of the manuscript, and Louella Dolar constructed the figures from the original data of the authors.
REFERENCES
Adams, N.J. & Klages, N.T. 1987. Seasonal variation in the diet of the King Penguin (Aptenodytes patagonicus) at sub-Antarctic Marion Island. Journal of Zoology (London) 212: 303-324.
Ancel, A., Kooyman, G., Ponganis, P. J., Gendner, P. J., Lignon, J. P., Mestre, J., Huin, X., P.H.Thorson, Robisson, P. & Maho, Y. L. 1992. Foraging behaviour of Emperor Penguins as a resource detector in winter and summer. Nature 360: 336-339.
Barrat, A. 1976. Quelques aspects de la biologie et de l'ecologie du manchot royal (Aptenodytes patagonicus) des Isles Crozet. Comite National Francais des Recherches Antarctiques 40: 9-51.
Bost, C.A., Georges, J.Y., Guinet, C., Cherel, Y., Puetz, K., Charrassin, J. B., Handrich, Y., Zorn, T., Lage, J. & Le Maho, Y. 1997. Foraging habitat and food intake of satellite-tracked King Penguins during the austral summer at Crozet Archipelago. Marine Ecology Progress Series 150: 21-33.
Chappell, M.A., Shoemaker, V.H., Janes, D.N., Bucher, T.L. & Maloney S.K. 1993. Diving behaviour during foraging in breeding Adelie Penguins. Ecology 74: 1204-1215.
Cherel, Y. & Kooyman, G.L. 1998. The food of Emperor Penguins (Aptenodytes forsteri) in the western Ross Sea, Antarctica. Polar Biology. Polar Biology 130: 335-344.
Cherel, Y., Ridoux, V. & Rodhouse, P.G. 1996. Fish and squid in the diet of King Penguin chicks, Aptenodytes patagonicus, during winter at sub-antarctic Crozet Islands. Marine Biology (Berlin) 126: 559-570.
Croll, D. & Tershy, B. 1998. Penguins, fur seals, and fishing: prey requirements and potential competition in the South Shetland Islands, Antarctica. Polar Biology 19: 365-374.
Croxall, J., Prince, P. & Reid, K. 1997. Dietary segregation of krill-eating South Georgia seabirds. Journal of Zoology (London) 242: 531-56.
Croxall, J.P., Briggs D.R., Kato, A., Naito, Y., Watanuki, Y. & Williams, T.D. 1993. Diving pattern and performance in the Macaroni Penguin, Eudyptes chrysolophus. Journal of Zoology (London) 230: 31-47.
Croxall, J.P. 1984. Seabird ecology. In: Laws, R.M. (ed) Ecology of the Antarctic. London; Academic Press: 533-619
Croxall, J.P. & Prince, P.A. 1980. Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biological Journal of the Linnean Society 14: 103-131.
Davis, L.S., Boersma, P.D. & Court, G.S.. 1996. Satellite telemetry of the winter migration of Adelie penguins (Pygoscelis adeliae). Polar Biology 16: 221-225.
Davis, L.S. & Miller, G.D. 1992. Satellite tracking of Adelie Penguins. Polar Biology 12: 503-506.
Emison, W. B. 1968. Food preferences of the Adelie Penguin at Cape Crozier, Ross Island. Washington, D.C., American Geophysical Union 12: 191-212 .
Fraser, W.R. &Trivelpiece, W.Z. 1996. Factors controlling the distribution of seabirds: winter-summer heterogeneity in the distribution of Adelie Penguin populations. In: Ross, R.M., Hofmann, E.E. & Quetin, L.B. (eds) Foundations for ecological research west of the Antarctic peninsula. Washington, D.C., American Geophysical Union. 70: 257-272.
Guinet, C., Khoudil, M., Bost, C. A., Durbec, J. P., Georges, J. Y., Mouchot, M. C. & Jouventin, P. 1997. Foraging behaviour of satellite-tracked king penguins in relation to sea-surface temperatures obtained by satellite telemetry at Crozet Archipelago, a study during three austral summers. Marine Ecology Progress Series 150: 11-20.
Handrich, Y., Bevan, R.M., Charrassin, J.B., Butler, P.J., Puetz, K., Woakes, A.J. Lage, J.B. & Le Maho, Y. 1997. Hypothermia in foraging King Penguins. Nature (London) 388: 64-67.
Hindell, M.A. 1988. The diet of the Royal Penguin Eudyptes schlegeli at Macquarie Island. Emu 88: 219-26.
Hull, C. 1997. The comparative foraging ecology of Royal Eudyptes schlegeli and Rockhopper E. chrysocome penguins. Ph.D. thesis. Hobart: University of Tasmania.
Hull, C.L., Hindell, M.A. & Michael, K. 1997. The foraging zones of royal penguins during the breeding season, and their association with oceanographic features. Marine Ecology Progress Series 153: 217-228.
Jablonski, B. 1985. The diet of penguins on King George Island, South Shetland Islands. Acta Zool. Cracov 29:117-186.
Jouventin, P., Capdeville, D., Cuenot-Chaillet, F. & Boiteau, C. 1994. Exploitation of pelagic resources by a non-flying seabird: Satellite tracking of the King Penguin throughout the breeding cycle. Marine Ecology Progress Series 106: 11-19.
Kerry, K.R., Clarke, J.R. & Else, G.D. 1996. The foraging range of Adelie Penguins at Bechervaise Island, Mac. Robertson Land, Antarctica as determined by satellite telemetry. In: Dann, P.I.N. & Reilly, P. (eds) The penguins: ecology and management. Second International Penguin Conference; Cowes, Victoria, Australia; August 1992. Chipping Norton, New South Wales, Australia: Surrey Beatty and Sons Pty Ltd: 216-243.
Kirkwood, R. & Robertson, G. 1997a. The foraging ecology of female Emperor Penguins in winter. Ecological Monographs 67: 155-176.
Kirkwood, R. & Robertson, G. 1997b. Seasonal change in the foraging ecology of Emperor Penguins on the Mawson Coast, Antarctica. Marine Ecology Progress Series 156: 205-223.
Klages, N. 1989. Food and feeding ecology of emperor penguins in the eastern Weddell Sea. Polar Biology 9: 385-390.
Kooyman, G.L. 1989. Diverse divers: physiology and behaviour. New York; Springer-Verlag: 216pp.
Kooyman, G.L., Cherel, Y., Le Maho, Y., Croxall, J.P., Thorson, P.H., Ridoux, V. & Kooyman C.A. 1992a. Diving behaviour and energetics during foraging cycles in King Penguins. Ecological Monographs 62: 143-163.
Kooyman, G.L., Croll, D., Stone, S. & Smith, S. 1990. Emperor Penguin colony at Cape Washington, Antarctica. Polar Record 26: 103-108.
Kooyman G.L., Ponganis P.J, Castellini, M.A., Ponganis, E.P., Ponganis K.V, Thorson, P.H., Eckert S.A. & Le Maho,Y. 1992b. Heart rates and swim speeds of Emperor Penguins diving under sea ice. Journal of experimental Biology 165: 161-180.
Kooyman, G. L. & Kooyman, T. G. 1995. Diving behaviour or Emperor Penguins nurturing chicks at Coulman Island, Antarctica. Condor 97: 536-549.
Kooyman, G. L., Kooyman, T. G., Horning, M. & Kooyman, C. A. 1996. Penguin dispersal after fledging. Nature 383: 397.
Lishman, G. S. 1985. The food and ecology of Adelie Penguins (Pygoscelis adeliae) and Chinstrap penguins (P. antarctica) at Signy Island, South Orkney Islands. J. Zool., Lond. 205: 245-263.
Olsson, O. 1996. Seasonal effects of timing and reproduction in the King Penguin: A unique breeding cycle. Journal of Avian Biology 27: 7-14.
Olsson, O. & North, A.W. 1997. Diet of the King Penguin Aptenodytes patagonicus during three summers at South Georgia. Ibis 139: 504-512.
Putz, K. 1995. The post-moult diet of Emperor Penguins (Aptenodytes forsteri) in the eastern Weddell Sea, Antarctica. Polar Biology 15: 457-463.
Putz, K. & Bost, C.A. 1994. Feeding behaviour of free-ranging King Penguins (Aptenodytes patagonicus). Ecology 75: 489-497.
Robertson, G. & Newgrain, K. 1996. The food and energy intake rates of adult Emperor Penguins (Aptenodytes forsteri) rearing chicks. Antarctic Science 8: 37-44.
Robertson, G., Williams, R., Green, K. & Robertson, L. 1994. Diet composition of emperor penguin chicks Aptenodytes forsteri at two Mawson Coast colonies, Antarctica. Ibis 136: 19-31.
Sadleir, M.F.S. & Lay, K.M. 1990. Foraging movements of Adelie penguins (Pygoscelis adeliae) in McMurdo Sound. In: Davis, L.S. & Darby, J.T. (eds). Penguin biology. San Diego; Academic Press: 157-179.
Stonehouse, B. 1956. The King Penguin of South Georgia. Nature 178: 1424-1426.
Stonehouse, B. 1960. The King Penguin of South Georgia I. Breeding behaviour and development. F.I.D.S. Scientific Reports No. 23:1-81.
Trathan, P., Murphy, E., Croxall, J.P. & Everson, I. 1998. Use of at-sea distribution data to derive potential foraging ranges of macaroni penguins during the breeding season. Marine Ecology Progress Series 169: 263-275.
Trivelpiece, W.Z.,Trivelpiece, S.G. & Volkman N.J. 1987. Ecological Segregation of Adelie, Gentoo, and Chinstrap Penguins at King George island, Antarctica. Ecology 68: 351-61.
Volkman, N., Presler, P. and Trivelpiece, W. 1980. Diets of pygoscelid penguins at King George Island, Antarctica. Condor 82: 373-8.
Watanuki, Y., Kato, A., Mori, Y. & Naito, Y. 1993. Diving performance of Adelie Penguins in relation to food availability in fast sea-ice areas: comparison between years. Journal of Animal Ecology 62: 634-646.
Wienecke, B.C. and Robertson, G. 1997. Foraging space of Emperor Penguins Aptenodytes forsteri in Antarctic shelf waters in winter. Marine Ecology Progress Series 159: 249-63.
Williams, T.D. 1991. Foraging ecology and diet of Gentoo Penguins (Pygoscelis papua) at South Georgia during winter and an assessment of their winter prey consumption. Ibis 133: 3-13.
Williams, T. D. 1995. The penguins. Oxford; Oxford University Press.
Wilson, R., Alvarrez, B., La Torre, L., Adelung, D., Culik, B. & Bannasch, R. 1998. The movements of Gentoo Penguins Pygoscelis papua from Ardley Island, Antarctica. Polar Biology 19: 407-413.
Woehler, E.J. 1993. The distribution and abundance of Antarctic and Subantarctic penguins. Cambridge; SCAR: 76pp.
Table 1. Summary of species habitat in the breeding season.

Fig. 1. Timing of foraging, breeding, and moult of Macaroni, Royal, Adelie, King and Emperor Penguins. There is variation in the cycle depending on latitude of the colony location. The 'ideal' King Penguin cycle means the timing that will be most likely successful in fledging a chick. This cycle begins with laying in early January and ends with fledging in late December. Such successful breeders start late for their next breeding effort (* and broken line indicating end of the first cycle), and most likely fail because laying occurs too close to the winter departure of the parents, and the recently hatched chick is unable to endure the extended fast. Adelie and Emperor cycles may be later with higher latitude. The Emperor cycle depicted is for a latitude of about 73o S; this is about the middle of the latitudinal range of this species. Abbreviations: WS=winter at sea; B=courting and breeding; L=egg laying; I=incubation; H=hatching; G=guard; C=creche; F=fledging; M=moult.
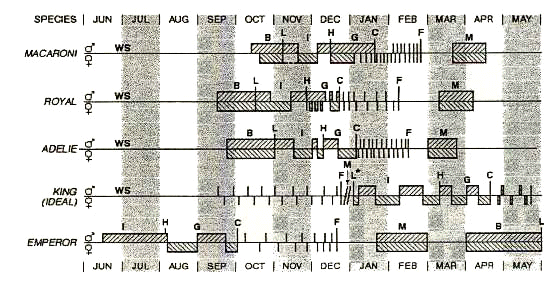
Fig. 2. Royal Penguin travel from Macquarie Island during different stages of reproduction as indicated by the symbols: squares=female incubation; triangle=male incubation; circle=male guard; diamond=female guard; diamond=female creche. Bathymetry isobaths are: short dashes=1000m; long dashes=3000m; solid=4000m (after Hull, et al. 1997).
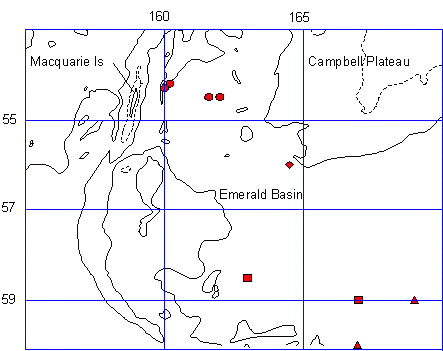
Fig. 3a. King Penguin travel from Possession Island, Crozet Archipelago during different stages of chick nurturing from incubation to final growth. Bathymetry isobaths are: long dashes=3000m; solid line=4000m (after Jouventin et al. 1994).
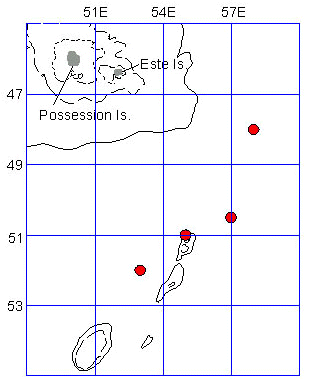
Fig. 3b. King Penguin travel from South Georgia during early chick development from February to March. Bathymetry isobaths are: dots=200m; short dashes=1000m; long dashes=3000m; solid line=4000m (Olsson, unpub observ).
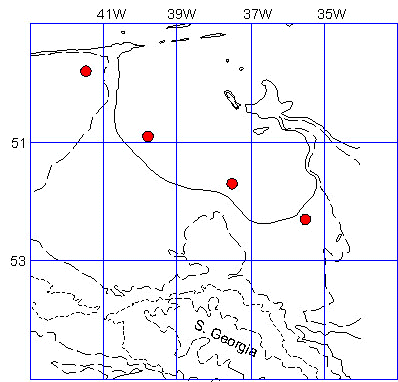
Fig. 4. King Penguin diving pattern in the twilight zone. The Y axis is local time (after Kooyman, et al. 1992).
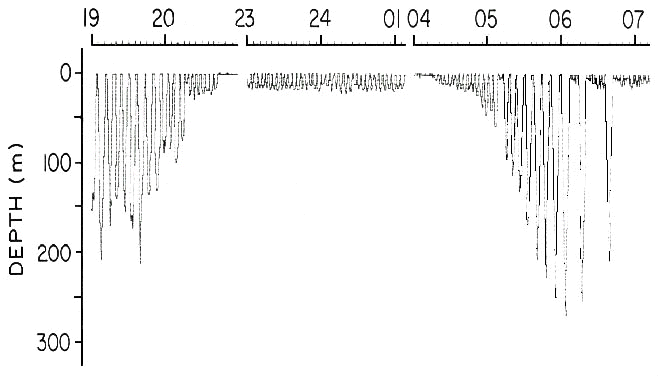
Fig. 5. Adelie Penguin tracks from Bechervaise Island during different stages of reproduction. Diamonds are incubation; closed circles are guard and creche stages. Bathymetry isobaths are: short dashes=1000m; long dashes=3000m (after Kerry et al. 1995).
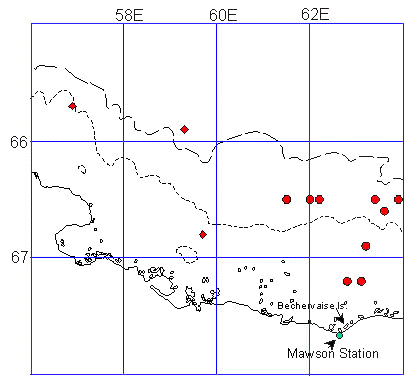
Fig. 6a. Emperor Penguin favoured foraging areas from Auster colony, Antarctica from winter through spring. The preferred foraging areas are hatched. Bathymetry isobaths are: short dashes=1000m; long dashes=3000m (after Kirkwood & Robertson, 1997; Wienecke & Robertson, 1997).
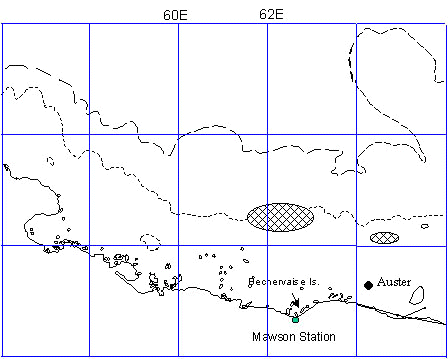
Fig. 6b. Emperor Penguin travel from Coulman Island, Ross sea, Antarctica, during November chick nurturing. Bathymetry isobaths are: long dashes=500m; short dashes=1000m (Kooyman, unpub observ).
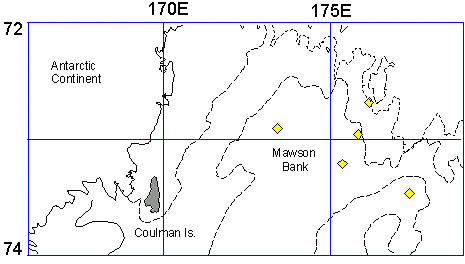
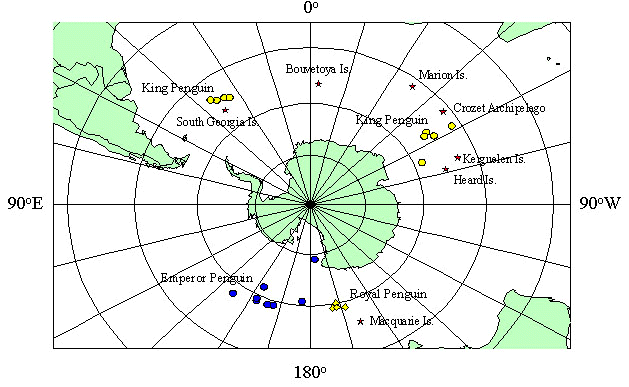
Fig. 7. Summary of foraging location while nurturing chicks of Royal and King Penguins, and the fall and winter distribution from the colony of a King Penguin adult, and Emperor Penguin juveniles. Stars indicate location of sub-antarctic islands; open circles are the maximum distance of King Penguin foraging trips from South Georgia and Crozet Archipelago. One exception is the most distant location from Crozet of a winter trip. Diamonds are the most extensive trips of Royal Penguins; solid circles are the late February early March positions of juvenile Emperor Penguins (Kooyman, et al. 1996).