S37.4: Energy and nutrient utilisation efficiencies in birds: A review
Franz Bairlein
Institut für Vogelforschung ‘Vogelwarte Helgoland’, An der Vogelwarte 21, D-26386 Wilhelmshaven, Germany; fax 49 4421 968955, e-mail bairlein@ifv-terramare.fh-wilhelmshaven.de
Bairlein, F. 1999. Energy and nutrient utilisation efficiencies in birds - A review. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2221-2246. Johannesburg: BirdLife South Africa.Ingested (consumed) food is digested, absorbed and metabolized. The amount of food that must be consumed to meet the requirements is related to the losses in digestion and metabolism. Only part of the food can be utilised. Utilisation efficiency (assimilation efficiency) which is the relative amount of ingested matter or energy that does not appear in the faeces is, therefore, an import factor to assess the daily food requirements in animals. The quantification and understanding of the efficiencies of nutrient utilisation is basic in food studies. The paper will compile current information on nutrient utilisation efficiencies in birds, and analyse them in relation to taxon, body size (metabolic rate), food type, nutrient composition of food, plant and insect chemical defences, dietary specialisation, food intake, mode of feeding, temporal patterns, environmental temperature and photoperiod, and the specific requirements related to physiological state (e.g. reproduction, moult, migration, hunger), growth and development. Utilisation efficiency is not a simple aspect. Rather it shows extensive variation ranging from less than 20 % in some herbivores to almost 100 % in nectarivores. The review will illustrate the complexity, and how important it is to learn more about the processes after a food has been swallowed.
INTRODUCTION
Most work on the nutrition of wild birds has simply described what they eat and the preferences they show for different foods among those available. There is, however, also a great deal more to be learned about what happens within the bird after it has fed (Clench & Mathias 1992). Not all of the food that is eaten by individuals can be utilised and is available for metabolism due to the inefficiencies in the digestive process. The amount of food that must be consumed to meet the requirements is related to the losses in digestion and metabolism. A measure of assimilation efficiency, which is the relative amount of ingested matter or energy that does not appear in the excreta, is essential for accurate conversion of food intake into the metabolic requirements of individuals. The quantification and understanding of the efficiencies of energy and nutrient utilisation is basic in food studies.
Digestive efficiency is of ecological importance because it mediates the birds’ interactions with the environment (Karasov 1990). A more thorough consideration of digestive efficiency is, therefore, important in assessing the control of food intake in birds (Denbow 1985), in the evaluation of digestion optimisation models (McWilliams & Karasov 1998) and to predict foraging decisions in animals. Data on digestion efficiencies are even needed in bio-energetic models (Furness 1978, Brugger 1993).
Consequently, there is a bulk of information on the efficiency of assimilation of food consumed by birds which has already been reviewed by Kendeigh et al. (1977), Castro et al. (1989b), Karasov (1990), and Barton & Houston (1993) for birds of prey. Likely stimulated by these reviews, even more data became available since then which makes it worthwhile to summarise again. While Castro et al. (1989b) compiled approximately 150 single cases of energy utilisation coefficients, and Karasov (1990) approximately 250 digestion trials, the current review is based upon more than one thousand cases of assimilation efficiencies reported in the literature.
The paper will compile these current information on energy and nutrient utilisation efficiencies in birds, and analyse them in relation to taxon, body size, metabolic rate, food type, nutrient and chemical composition of food, plant and insect chemical defences, food intake, temporal patterns, environmental temperature and photoperiod. Utilisation efficiency is neither a simple nor invariant parameter. Rather it shows extensive variation ranging from less than 20 % in some herbivores to almost 100 % in nectarivores. The review will illustrate this variation and how important it is to learn more about the processes after a food has been swallowed, and it aims to stimulate further studies into the mechanisms of digestion and absorption of energy and nutrients by birds.
Terminology and methods to calculate assimilation efficiency
There is a considerable diversity of terms used to describe the proportion of ingested food that is converted into usable energy or nutrients (c.f. Castro et al. 1989, Karasov 1990, Robbins 1993, Klasing 1998). ‘Assimilation efficiency’, ‘utilisation efficiency’, ‘digestive efficiency’, ‘coefficient of metabolic utilisation’, ‘metabolizability coefficient’ or ‘digestibility’ among others are used as synonyms. In any case, it describes the fraction of matter, energy or nutrients of the diet that is digested. It does not reflect the fraction which is incorporated into body tissue.
In birds, undigested food and urinary endogenous losses are intimately mixed in the cloaca, and the faeces may include microbial products. This leads to under-estimates of the actual digestion of food matter (Robbins 1993). Consequently, assimilation efficiency in birds based on the difference in matter between intake and excretory loss can only be termed ‘apparent’ because it is not corrected for the endogenous losses. This is particularly important in birds that loose mass because assimilation efficiency can be under-estimated due to endogenous losses (Blem 1976). A further underestimate is possible due to fecal uric acid. Krebs & Avery (1984) reported on estimates of assimilation efficiency about 2-5% too low without accounting for uric acid. The extraction of uric acid from the faeces is particularly important in the calculation of nitrogen digestibility rates. In the Hoatzin Ophistocomus hoazin, nitrogen digestibility without uric acid extraction was 56 %, while uric acid extraction showed a nitrogen digestibility of 78 % (Grajal 1995). The importance of correcting for nitrogen retention has long been recognised in poultry science (Sibbald 1980). Nitrogen correction is particularly important when working with birds that have a nitrogen retention different from zero, such as growing or fasted birds. However, this has often been neglected in assimilation efficiency studies. In Kittiwakes Rissa tridactyla and Guillemots Uria aalge, correction of assimilation efficiencies for nitrogen retention reduced the assimilation efficiencies by 2-7% (Brekke & Gabrielsen 1994).
There are two principal methods for calculating assimilation efficiency. The direct method involves the use of captive birds in which the food intake and food content, and excreta production and composition are directly measured over a period of time. The amount of food metabolised is calculated by subtracting the weight, energy or nutrient content of the resulting faeces and excreta from that of food eaten. Metabolizability in then obtained by dividing that quantity by the weight, energy or nutrient content of food consumed.
The other method is an indirect one using an inert recognisable marker (tracer). The ideal tracer should not be digestible, nor should it be added to the digesta from endogenous sources. Then, assimilation efficiency can be estimated by the percent tracer in the food and the excreta (Karasov 1990), dividing the concentration of the marker in the food by the concentration of the marker in the faeces and subtracting the results from 1. Digestibility estimates using non-digestible markers gave good approximation of the total collection method (e.g. in Rock Ptarmigan Lagopus mutus; Gasaway et al. 1976).
In captivity, tracers can be added to the food. Tracers used in captive studies were, for example, pieces of plastic, rubber, glass beads, carmin-red, chromium oxide, Cr-EDTA, ferric oxide, Ce-144, titanium dioxide, or various radio-labelled substances, e.g. 3H-polyethylenglycol (for review see Robbins 1993).
In free-ranging birds, the most common method used in the analysis of assimilation efficiency is the use of naturally occurring tracers, e.g. minerals, lignin, cellulose or plant pigments. In herbivores, the use of plant pigments for estimating apparent assimilation efficiency is attractive because the technique is in-expensive, relatively straightforward, and is not time consuming (e.g. Boudewijn 1984, Lane & Hassall 1996). Studies showed good recovery of plant pigments in the faeces (Drent et al. 1978), and circumstantial evidence that chlorophylls might not be digested by geese (Buchsbaum et al. 1986). However, the chromogen marker method has the disadvantage in a field situation that the plant pigments are mutable and that the faecal material must be collected and frozen within an hour of production for the technique to be accurate (Lane & Hassall 1996). It is also inappropriate in circumstances where geese forage on plant material which is low in chromogen, such as seeds. Further problems arise if birds may digest cellulose or lignin to some degree. There is some evidence, that both cell wall contents are subject to partial degradation or alteration during passage through the gastrointestinal tract (see below). An important consideration when measuring assimilation efficiency indirectly with naturally occurring markers is how food samples are collected (Lane & Hassall 1996). Foragers may be selective (Peterson & Wunder 1997) so that variation in the marker between different foods will probably lead to erroneous results if foods are not sampled in a manner representative of the way the birds graze. Therefore, food plants must be sampled ‘realistically’ (Lane & Hassall 1996).
Besides in vivo methods, in vitro methods are used to study organic matter digestibility of food mimicking the gastric juices. Food samples are treated in a water bath at body temperature with pepsin or ruminal fluids (e.g. Brugger 1992, Grajal 1995; for review see Robbins 1993). In the Hoatzin, in vitro organic matter digestibility did not differ significantly from the average organic matter digestibility of the experimental diets by captive birds (Grajal 1995), but there may be considerable variation in estimates because there is extensive interlaboratory variation in techniques and results (Robbins 1993). In vitro estimates have not been compiled for this study, although they may be a reliable tool to assess the biological value of a food.
Material and Methods
Data on assimilation efficiencies of dry matter, energy, nutrients and non-nutrients were compiled from literature. If available the type of food and the energy, nutrient, ash and fibre content of the diet were recorded, as it was with age and body mass of the experimental birds. Furthermore, it was noted whether the study was carried out in captivity or in the wild, if the data are derived from total collection trials or by using a marker. Moreover, any other information on a trial (season, ambient temperature, photoperiod) was compiled. Body mass was either taken from the studies or from Dunning (1993). In order to scale assimilation efficiencies to metabolic demands, basal metabolic rate (BMR) and daily energy expenditure (DEE), were calculated, following the equations given by Ricklefs et al. (1996).
The great majority of the compiled cases reported on energy assimilation coefficients, but approximately 23% did so only for dry matter assimilation. In 129 cases, however, both coefficients were reported. Energy assimilation efficiencies were highly correlated to dry matter digestibilties (r = 0.879, p = 0.000). Therefore, dry matter assimilation coefficients have been converted into energy assimilation coefficients using the food type specific regressions in order to increase sample size for further analyses. There was no significant difference in energy assimilation coefficients of the derived ones and the ones calculated without the derived cases (Mann-Whitney U-test: p >> 0.05).
Due to space limitations, the complete list of data gathered nor the many sources cannot be presented. A list of all references used is available on request.
RESULTS
1151 cases of 184 different species of 60 different families belonging to 24 different orders were compiled. 206 cases were reported without age, 799 cases of adult birds and 146 cases of nestlings and chicks. 20 cases were reported from free-living birds. Average assimilation coefficients were neither different between the cases with unknown age as compared to the group of adults nor between free-living birds and captive trials. Therefore, for further analyses, the group of cases with unknown age were combined with the adult group as well as captive and field trials.
Further analyses and statistics were hampered by several shortcomings of the data. In many cases just a single record of a species or of a food type is available, or sample sizes are very small. In many cases, the experimental conditions, the status of the birds, or details about the food are missing. A few taxonomic groups dominate the data. 40% (n = 71) of the species and 47% of the records are belonging to Passeriformes, 12% of species and 11% of records to Anseriformes, and 10% of species and 20% of records to Galliformes. Moreover, owing to the many captive studies, artificial food dominates the food types used in the trials. 34% of all records are from trials with artificial food.
Assimilation efficiencies of adult birds
The influence of taxon and food on energy assimilation
Kendeigh et al. (1977), Castro et al. (1989b) and Karasov (1990) found significant relationships between energy assimilation efficiency and the type of food eaten, whereas the influence of taxon was less clear.
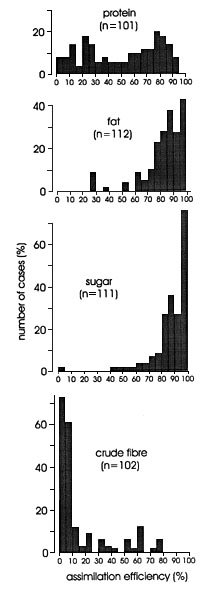
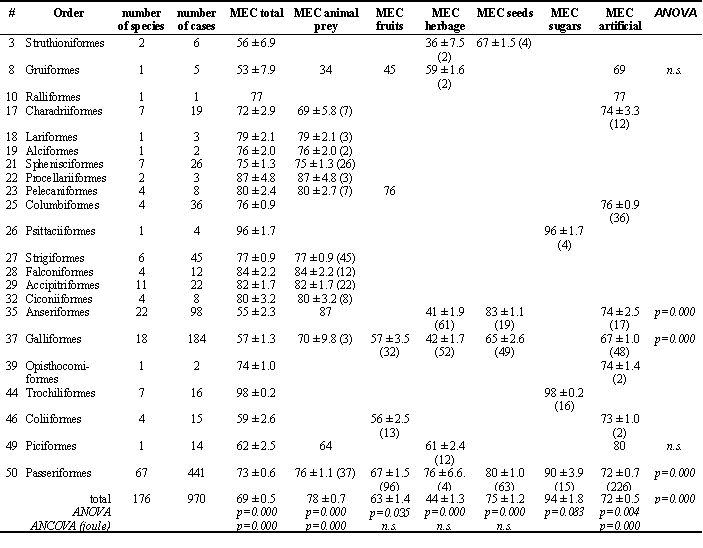
Fig. 1 shows pronounced differences in the frequency distribution of assimilation coefficients for major food groups. Whereas estimates of energy assimilation coefficients for sugars and for animal prey are rather narrowly grouped, estimates for seeds, fruits and herbage as well as for artificial diets show much more variation. The major food groups are significantly different (ANOVA: F = 141.5, p = 0.000) with lowest estimates for herbage, and highest for sugars (Table 1).
The difference in average assimilation rates between food groups is not an effect of taxon. Within the taxa eating different foods and with appropriate sample sizes, the general difference between the food groups remains (Table 1).
There is a further differentiation within some of the major food groups. For animal prey, species feeding on birds exhibit the highest estimate of assimilation efficiency, the lowest is obtained by vultures feeding on bones (Table 2).
Considering taxon, analysis of variance among all data revealed a highly significant effect of taxon (ANOVA: F = 17.96, p = 0.000; Table 1). Apart from sugars, these differences even hold within the major food groups (Table 1), showing that there is evidence that some phylogenetic differences exist.
The role of food intake in energy assimilation
It has often been discussed whether energy assimilation efficiency is affected by the amount of food ingested (Sibly 1981, Robbins 1993). There is a number of studies showing some relationships between food intake or meal size and energy assimilation efficiency, but the patterns are not consistent. In three species of granivorous songbirds, Meienberger & Ziswiler (1990) found a positive relationship between energy assimilation efficiency and daily food intake for a protein poor diet (9% protein) whereas no such relationship appeared for a protein rich diet (15% protein). Similar results were obtained in Common Bulbuls Pycnonothus barbatus (Mlingwa 1997). Energy assimilation efficiency increased with food intake in Great Blue Herons Ardea herodias feeding on mackarel and trout, but no consistent pattern was found in a herring diet (Bennett & Hart 1993). In Ruffed Grouse Bonasa umbellus, Guglielmo & Karasov (1993) found a non-linear positive relationship between energy utilisation efficiencies and food intake. In American Robins Turdus migratorius and European Starlings Sturnus vulgaris feeding on crickets daily consumption dropped as assimilation efficiency increased (Levey & Karasov 1989). A negative relationship between energy assimilation efficiency and food intake was also found in Whimbrels Numenius phaeops feeding on fiddler crabs (Zwarts & Blomert 1990). In Garden Warblers Sylvia borin, both dry matter and energy utilisation efficiencies decreased with daily food intake in birds that were not in the seasonal phase of migratory fattening, whereas the latter birds showed a tendency to increase utilisation efficiencies with increasing food intake (Hume & Biebach 1996). In Cardinals Cardinalis cardinalis and Song Sparrows Zonotrichia melodia (Willson & Harmeson 1983) as well as in three species of sub-Antarctic seabirds (Jackson & Place 1990) energy utilisation efficiencies were not affected by food intake.
For most studies compiled for the present review, data on food intake were not available. In order to assess the effect of food intake on assimilation efficiency, BMR and DEE estimates were calculated as measures of food intake. In general, food intake is closely linked to these predicted metabolic requirements (Daan et al. 1990, 1991, Lindström & Kvist 1995). Correlation analysis among all trials showed slight but highly significant negative relationships between energy assimilation efficiency and both metabolic parameters, (BMR: r = -0.147, p = 0.000; DEE: r = -0.151, p = 0.000; n = 965 each). No significant effects of food type were found on these relationships, while there is evidence for differences among taxa (Table 3). In some taxa, the relationship between assimilation efficiency and DEE is significantly negative, while in other it is significantly positive. The casual relationships need to be evaluated.
The role of nutrients in energy assimilation
Dietary nutrient content and nutrient composition can influence energy assimilation efficiency of birds (Karasov 1990, Robbins 1993), however, only few studies evaluated the relationship properly.
For dietary protein there is a trend towards increased energy assimilation efficiency with increased protein content (Martin 1968, Bairlein 1987, Meienberger & Ziswiler 1990, Eggel 1991, Murphy 1993). A similar relationship was found in in vitro studies of digestibility of geese and ptarmigan food (Moss et al. 1974, Aerts et al. 1996). However, there is evidence for differences between species, and for proteins differing in quality. In a comparative study using identical experimental regimes for Zebra Finch Teaniopygia guttata, Java Sparrow Padda oryzivora, and White-Backed Mannikin Lonchura striata (Meienberger & Ziswiler 1990), Zebra Finches continuously increased energy assimilation efficiency between 4% and 15% dietary protein, while Java Sparrows increased towards 10% dietary protein and sharply decreased energy assimilation efficiency at higher protein contents. White-Backed Mannikin did not change energy assimilation efficiency with increasing dietary protein content. Protein quality is a another possible factor for differences in assimilation efficiencies between foods (McNab & Shannon 1974, Karasov 1990). In four species of fruit doves, Eggel (1991) found a significant lower energy assimilation efficiency for plant protein diets than for animal protein diets (74.6 ± 5.6%; n = 44 vs. 79.6 ± 2.7%; n = 106; Mann-Whitney U-test: p = 0.001). This difference was especially pronounced in low protein diets (3% protein) where the plant protein diet showed an energy assimilation coefficient of just 67.7 ± 6.6% (n = 7) compared to 79.5 ± 2.4% (n = 18) for the animal protein diet. In Tree Sparrows Spizella arborea, the efficiency of energy utilisation was significantly lower for birds receiving low-lysine food than for control birds (Parrish & Martin 1977). For bobwhite food, the amino acid profiles indicated foraging quality better than crude protein estimates (Peoples et al. 1994).
The fat content of food can also influence energy assimilation efficiencies, but there is no consistent pattern (Mateos & Sell 1981, Castro et al. 1989a). While some species were found to increase assimilation efficiency with increasing levels of supplementary fats to diets, other decreased the rate of use with extra fat, or did not respond. In poultry, assimilation efficiency improved higher than expected after supplementing their foods with fats. This may be caused by a synergism between saturated and unsaturated fatty acids (Mateos & Sell 1980). Fatty acids from fish are both saturated and unsaturated. Therefore, it is possible that fat from fish shows this ‘extra calorific effect’ in seabirds assimilation efficiency (Jackson & Place 1990). In Kittiwakes and Brünnich’s Guillemots Uria lomvia, energy assimilation efficiency increased with increasing fat contents of foods (Brekke & Gabrielsen 1994). Renner & Hill (1961) and Clifford et al. (1986) showed that absorbability of fatty acids depends on the degree of unsaturation, and decreases with increasing saturation. Lipids may enhance energy utilisation of food by slowing intestinal motility and rate of passage (Duke & Evanson 1972, Roby et al. 1986, Clench & Mathias 1992). However, in American Kestrels Falco sparverius, fat content of the diet had little effect on either the gastrointestinal gross anatomy and contractile activity (Duke et al. 1997). Lean meat diets used in raptors may have low metabolizability of energy because of their high protein and comparatively low fat contents (Kirkwood 1979, Castro et al. 1989).
In the current analysis, energy assimilation efficiency was positively correlated with the energy and the sugar content of the diet, and negatively correlated with the fat and the fibre content; no relationship was found to the protein content and the ash content (Table 4). However, there are pronounced differences between the major food groups (Table 4). Considering these dietary nutrient relationships in an analysis of co-variance for the analysis of taxonomic contrasts within the food groups showed that the statistical differences between taxa disappeared for fruits, herbage and seeds (Table 1), but remained for animal prey and artificial diets. Thus, the apparent taxonomic differences in frugivores, herbivores and granivores were due to differences in nutrient and energy content of the foods. However, one should consider the small sample sizes and low taxonomic representativeness in these groups.
The effect of non-nutrients on energy assimilation: lignin, cellulose, chitin, plant secondary compounds
It is very recognised that there is an inverse relation between crude fibre content and digestibility of food in many herbivores (e.g. Moss 1977, Drent et al. 1978/79, Zbinden 1980, Moss & Trenholm 1987, Robbins 1993, Hupp et al. 1996, Klasing 1998). Assimilation efficiencies of energy and nutrients are consistently lower in birds fed high fibre food. The physiological basis for the inhibition of digestiblity by the complex of substances separated as ‘crude fibre’ (chiefly cellulose and lignin) is poorly understood (Robbins 1993).
Much less is known about the effect of chitin in diets of insectivores, although it is known that chitin content of food influences diet selection in insectivores (Zack & Falls 1978). Metabolizable energy coefficient values lower than 70-80% values normally cited for insectivorous species are probably typical for birds that eat insects with heavy exoskeletons (Krebs & Avery 1984, Koenig 1991).
Plant secondary compounds are well known to affect diet choice and digestion of food (for review see Robbins 1993, Klasing 1998, and the paper of Levey & Cipollini in this issue). Tannins in food can inhibit protein digestibility and contribute to a lower energy utilisation (Servello & Kirkpatrick 1989, Johnson et al. 1993, Nelson et al. 1996). They are also known to reduce glucose uptake by influencing the Na+ electro-chemical gradient, which provides the driving force for active glucose resorption (Welsh et al. 1989). In Acorn Woodpecker Melanerpes formicivorus, for example, energy assimilation efficiency varied according to the tannin content of the food (Koenig 1991), as it did in Ruffed Grouse (Servello et al. 1987, Guglielmo & Karasov 1995, Guglielmo et al. 1996). Considering the role of plant secondary compounds like tannins for assimilation efficiency, the question is whether they do reduce digestive efficiency per se or do they decrease utilisation efficiency due to detoxification costs (Karasov 1990). Moreover, reviewing the effects of tannins on food selection by mammalian herbivores, Mole & Waterman (1987) noted that there was little evidence to support the claim that tannins always effectively reduce digestibility. While high quantities of tannins are likely to have adverse consequences in precipitation of endogenous protein, low levels of tannins may even enhance protein digestibility (Mole & Waterman 1985, Bernays et al. 1989). The effects of non-tannin phenolics on digestion and absorption in birds have been largely ignored, although identified as important agents in ruminant herbivores (Iason & Murray 1996), as it is the case with other plant secondary compounds. In Ruffed Grouse, coniferyl benzoate decreased utilisation efficiencies by a dilution effect (Jakubas et al. 1993, Guglielmo et al. 1996). Plant secondary metabolites may even not only be detrimental for birds (Levey & Cipollini this issue). In some fruit-eating birds, addition of fruit secondary compounds increased consumption and digestion of artificial diets (Cipollini & Stiles 1993, Bairlein & Simons 1995, Bairlein 1996).
A yet almost completely unexplored aspect is the role of protein inhibitors on digestibilities and utilisation of dietary nutrients. Such protein inhibitors occur widely in plants, and have been shown to affect the efficiency of utilisation of dietary proteins and lipids in rats (Pusztai et al. 1995). In geese, a gracing-induced action of trypsin inhibitors may affect protein digestibility, as shown for lemmings (Seldahl et al. 1994).
Considering the effects of non-nutrients on energy assimilation efficiency in the current data set, only crude fibre and ash could be analysed because too little information was available for other food constituents. Among all trials, energy assimilation efficiency was strongly negatively correlated with fibre content of food, whereas there is no general trend concerning ash (Table 4), except for animal prey with a significantly negative effect of ash on energy utilisation efficiency. The non-significant correlation between food fibre and energy assimilation in the group of herbivores seems to contradict current knowledge on the relationship between plant fibre and digestion. However, this is not the case. Most studies have been carried out with artificial diets supplemented with fibre, and these trials are included in that group.
The assimilation of nutrients and other food constituents
Compared to the assimilation efficiency of dry matter or energy, much less is known about the efficiency of utilisation of the various nutrients and other food constituents.
Among all trials, protein utilisation efficiency averaged 51%, lipid utilisation 82% and soluble carbohydrate utilisation 87% (Table 5), but with considerable variation (Figure 2). Most variation appeared for protein. The rather low protein utilisation efficiencies are likely due to the inclusion of trials in which the experimental birds were in a negative nitrogen balance, thus endogenous nitrogen losses are included reducing the apparent assimilation coefficient. Analysis of variance among all cases showed significant differences in nutrient assimilation efficiency between major food groups and taxa for all nutrients (Table 6). The latter is very likely due to differences in diet, but data are too few to carry out more sophisticated analyses. Among the major food groups, protein and fat assimilation efficiency are highest for animal prey (Table 5).
Nutrient assimilation efficiency may be affected by the dietary nutrient content. In poultry, Sues (1974) reported an inverse relationship between lipid utilisation efficiency and the level of dietary fat, whereas Mateos & Sell (1981) found that apparent lipid digestibility increased with dietary fat content. In Garden Warblers, lipid utilisation efficiency was significantly higher with low fat diets than with high fat diets (89.4 ± 0.7% vs. 83.1 ± 0.8%; Mann-Whitney U-test: p < 0.001; Bairlein 1987). In fruit doves, lipid utilisation efficiency increased with increasing protein content of the diet, and it was significantly higher for animal protein diets than for plant protein diets (Eggel 1991).
Protein utilisation efficiency increased with increasing dietary protein content in Garden Warblers (Bairlein 1987). In these studies, undigested faecal protein was determined directly, so that endogenous losses of nitrogen did not influence the calculated assimilation coefficients. In four species of fruit doves, Eggel (1991) reported rather low protein assimilation efficiencies (on average 19.9 ± 1.2%; range of species averages: 12.9 to 28.7%), even if only birds maintaining body mass were considered. There was no consistent relationship to dietary protein content nor to dietary protein origin (plant vs. animal protein). In Whooping Crane Grus americana, protein digestibility for blue crab and rangia were higher than those for acorns and wolf berry (Nelson et al. 1996).
In Garden Warblers, dietary sugar content did not significantly influence sugar assimilation efficiency except that highest values were found in diets with very low soluble carbohydrate content (1% in dry matter: 92.7 ± 2.0% vs. 88.5 ± 0.9% for all diets, Mann-Whitney U-test: p < 0.001; Bairlein 1987). Concerning sugar diets, the types of carbohydrate, however, may influence sugar assimilation efficiency (e.g. Martinez del Rio et al. 1988, 1989, 1992, Martinez del Rio 1990, Karasov 1990, Karasov & Levey 1990, Jackson et al. 1998). This is particularly well known for sucrose. In birds lacking the enzyme sucrase, like some Sturnidae, no assimilation of sucrose is possible. In sunbirds, hummingbirds or honeyeaters, in contrast, sucrose assimilation is as high as with the other sugars. Considerable lower sucrose utilisation efficiencies (on average 61%) are reported from American and European songbirds. Also in the Rainbow Lorikeet Trichoglossus heamatodus glucose utilisation was significantly higher than sucrose assimilation efficiency (Karasov & Cork 1996). Comparatively low assimilation efficiency is mentioned for xylose, a pentose sugar which was recently discovered in the nectar of some Protaceae. Cape Sugarbirds Promerops cafer absorbed xylose much less (53 %) than the three major nectar sugars, sucrose, glucose, and fructose, with apparent absorption efficiencies of > 99 % (Jackson et al. 1998).
Plant and animal structural components like cellulose, lignin, and chitin, are most seen deterring the efficiency of assimilation. However, they are also subject to assimilation.
Crude fibre assimilation efficiency varied among food groups and taxa (Table 5 and Table 6). In Greylag Geese Anser anser, digestion of cellulose amounted to 17% for Scirpus litoralis and to 31% for Scirpus maritimus thus showing considerable differences between different plant species (Amat et al. 1991). High cellulose digestibilities were found in Snow Geese Chen caerulescens, with on average 44.6% for cellulose (Sedinger et al. 1995). Red Grouse Lagopus lagopus scoticus digested 38% of cellulose of its heather diet (Moss 1977), Brent Geese Branta bernicla 32% and Canada Geese Branta canadensis 18 to 30%, depending on food plant (Buchsbaum et al. 1986), and Barnacle Geese Branta leucopsis 26% (Prop & Vulnik 1992). Emus Dromaius novaehollandiae retained between 7 and 19% of the cellulose in experimental diets (Herd & Dawson 1984). The highest utilisation coefficient of cellulose yet estimated for birds is reported for the Hoatzin. Cellulose digestibility was 55% for a low-fibre diet and 63% for a high-fibre diet (Grajal 1995). Even lignin is subject to digestion in avian herbivores. Hoatzins digested 62% of lignin in a low-fibre diet and 39% in a high-fibre diet (Grajal 1995). Red Grouse retained 44% of lignin in heather (Moss 1977), and Snow Geese 7.8% (Sedinger et al. 1995). However, concerning the analysis of digestion of plant structural components, methodological pitfalls measuring fibre must be considered as fibre components may be partially soluble in chemical solutions used for analyses (for review see Robbins 1993) thus leading to erroneous estimates of cell wall assimilation.
Much less is know about digestibility of chitin. In Northern Bobwhites Colinus virginianus and American Robins feeding on crickets, apparent chitin digestion was 6.7% and 10%, respectively (Weiser et al. 1997). Much higher apparent chitin digestibilities have been reported in other birds. Japanese Nightingale Liothrix lutea digested 57% of the chitin in mealworms (Jeuniaux & Cornelius 1978), and several species of seabirds feeding on krill utilised between 39% (White-chinned Petrel Procellaria aequinoctialis) and 85% (King Penguins Aptenodytes patagonicus) of the chitin ingested (Jackson et al. 1992). In domestic chicken feeding on chitin added to their normal diets chitin digestibilities as high as 92% have been reported (Jeuniaux & Cornelius 1978, Hirona et al. 1984).
Also much less considered is the digestion of other food constituents such as waxes, though waxy prey is not uncommon. Waxes are present in many marine organisms, some bird species are know to feed on beeswax, e. g. the honey-guides, or some berries taken by birds are coated by waxes. Yellow-rumped Warbler Dendroica coronata and Tree-swallows Tachycineta bicolor feeding on wax-coated Myrica-berries digested 88% and 66% of the ingested bayberry wax, but only 56% and 19% of beeswax (Place & Stiles 1992). Black-throated Honey-guides Indicator indicator are apparently able to digest beeswax although assimilation coefficients are not reported (Diamond & Place 1988). Wax digestion is well reported in some seabirds (Obst 1986, Roby et al. 1986, Jackson & Place 1990). In three species of Southern Ocean seabirds, assimilation efficiencies of wax ester ranged between 45 and 90% and they were not significantly different from the assimilation of triglycerides, and Sooty Albatross Phoebetria fusca and White-chinned Petrel assimilated both lipids more efficiently than did Rockhopper Pengiuns Eudyptes chrysocome (Jackson & Place 1990).
Temporal changes in digestibility
Seasonal changes in assimilation efficiency have been reported for a number of bird species. Most are reported from calculations in free-living birds, thus they are likely due to changing food, as it is with Snow Goose (Bedard & Gauthier 1989), Brent Goose (Boudewijn 1984), Willow Ptarmigan Lagopus lagopus (West 1968) and Red Grouse (Moss & Parkinson 1972). However, there are few studies showing seasonal differences in assimilation also in captive trials with constant food conditions.
In outdoor captive Svalbard Ptarmigan Lagopus mutus and Willow Ptarmigan fed pellets, apparent dry matter assimilation efficiencies underwent seasonal variation, with highest levels in December (78 and 69%, respectively) and considerably lower levels in April (42% and 44%, respectively; Mortensen & Blix (1989). In captive European Kestrels Falco tinnunculus, winter (October to March) values of energy assimilation efficiency of common vole were significantly lower than summer (May to September) levels (66.7 vs. 70.4%; Masman 1986). This has been attributed to seasonal changes in ash content of voles due to changes in fur. However, in captive Long-eared Owls Asio otus fed with laboratory mice, energy assimilation efficiency also increased towards summer, with highest values in July to October (Wijnandts 1984). Five species of raptors kept indoor and fed day-old cockerels and measured twice in summer and winter exhibited a significant 3.2% higher digestive efficiency in summer (at 20oC) than in winter (at 0oC; Barton & Houston 1993). In contrast, in Rufous-collared Sparrow Zonotrichia capensis, apparent dry matter assimilation was less efficient in summer (May - September) than in December (77.4% vs. 83.4%; Novoa et al. 1996). Although tested in the laboratory, the experimental birds were captured in the wild immediately before the feeding trials. Thus, seasonal changing food in the wild could be responsible for the observed seasonal changes in assimilation efficiency.
Constant food, temperature and photoperiod regimes have been applied in studies with captive Garden Warblers (Bairlein 1985, Hume & Biebach 1996). Both studies revealed significant spontaneous seasonal differences in dry matter and energy assimilation efficiencies, with highest levels during seasonal fattening in preparation for migration (Table 7), which are not induced by temperature, photoperiod nor changing food. Concerning migratory fattening, changes in assimilation efficiencies have also been addressed by Merkel (1958) in Whitethroat Sylvia communis, Zimmermann (1968) in Dickcissel Spiza americana, Fry et al. (1972) in Yellow Wagtail Motacilla flava, and Bhatt & Chandola (1985) in Spotted Munia Lonchura punctulata, but no relationships between assimilation efficiencies and fattening were found in White-crowned Sparrow Zonotricha leucophrys (King 1961), Bobolink Dolichonix oryzivorus (Gifford & Odum 1965), and in Brent Goose (Bruns & TenThoren 1990), the latter birds, however, were kept outdoors.
A diurnal cyclic pattern of dry matter apparent digestibility is reported by Gasaway et al. (1976) in Rock Ptarmigan, with peaks of digestion near 1600 hours (darkness between 2300 and 0500 hours) and a decline throughout the night.
Transitional changes in assimilation efficiency are reported in response to dietary shifts. In Sanderlings Calidris alba feeding on Limulus-eggs, energy utilisation efficiency was significantly and positively correlated with days on the diet, while nitrogen balance did not change (Castro et al. 1989a). The birds obviously need some time to deal with the resistance of the egg cuticle to chemical and enzymatic digestion. European Starlings and American Robins significantly increased mass assimilation efficiency on a cricket diet by 9.6% and 15.1% from the first three days on the diet to the last three days of the 10 days trial (Levey & Karasov 1989). Neither mass nor energy utilisation coefficient changed significantly from the first to the last three days on a fruit diet, but mass utilisation efficiency dropped significantly from day 1 to day 2 (61% vs. 49%, p < 0.05) in the robins while it increased during the first three days on fruits in the starlings, although the difference between day 1 and day 3 was not significant (Levey & Karasov 1989). In Garden Warblers, switching food from an insect diet to a berry food resulted in changes in nutrient assimilation efficiencies which were different between the different nutrients (Simons 1991). While protein utilisation efficiency dropped to an almost constant lower level during the fruit diet, sugar assimilation efficiency slightly increased. Lipid utilisation efficiency dropped most in the first two days, but recovered to higher levels later during the fruit diet. Post-fruit values were similar to pre-fruit trials. In Common Bulbuls fed a low-protein diet dry matter assimilation efficiency increased during the first three days of trial and levelled thereafter (Mlingwa 1997).
Fasting may also affect assimilation efficiency. In four species of mousebirds, energy utilisation efficiencies increased during fasting by 6 to 46% compared to pre-fasting (Hoffmann & Prinzinger 1984). Garden Warblers which were food deprived for 2 days showed significantly higher dry matter and energy assimilation efficiencies on the first day of re-feeding than during the following days (83.4% vs. 78.2%; Hume & Biebach 1996).
Effect of photoperiod and temperature
Photoperiod and temperature can influence assimilation efficiency. Redpolls Carduelis flammea and Arctic Redpolls Carduelis hornemanni kept in short days (7 hours light) showed a significantly lower energy assimilation efficiency than birds kept on the same food in long days (24 hours light; 69.8 ± 0.9% vs. 71.9 ± 0.1%, Mann-Whitney U-test: p < 0.01; Brooks 1968). Similarly, six other songbird species kept in 10-hours days exhibited significantly lower energy utilisation efficiency compared to 15-hours days (81.5 ± 1.2% vs. 84.6 ± 1.1%, Mann-Whitney U-test: p = 0.042).
Concerning ambient temperature, no general relationship between assimilation efficiency and temperature emerged from the entire set of cases (r = 0.102, n = 204, p > 0.05), and there are several studies which failed to find such a relationship (Blem 1966, Brenner 1966, Martin 1968, Kontogiannis 1968, Kendeigh 1972), whilst others revealing some influence of ambient temperature on assimilation efficiencies in birds. In House Sparrow Passer domesticus, Kendeigh 1949, 1969) found a less efficient energy utilisation at lower temperatures (e.g. 83.8% at -31oC vs. 88.3% at 34oC). Also in House Sparrow, Davis (1955) reported on maximum energy utilisation rates at moderate temperatures 10-18oC), and lower values at lower or higher temperatures, irrespective of daylength. In Tree Sparrow, efficiency of energy utilisation of food steadily increased with increasing temperature, from 65% at -28oC to 77% at 37oC (West 1960). In Dark-eyed Juncos Junco hyemalis, White-throated Sparrows Zonotrichia albicollis, and House Sparrows, Seibert (1949) found lower energy assimilation efficiencies at -13oC compared to 22oC and 34oC, respectively, irrespective of photoperiod. In Cardinals and Song Sparrows the effect of temperature on energy utilisation efficiency depended on food: While utilisation efficiencies in Cardinals feeding sunflower seeds or ragweed or in Song Sparrows feeding on pigweed were higher at 26oC than at 0oC, the opposite was the case with foxtail, smartweed or hemp (Willson & Harmeson 1973). In Zebra Finches, food utilisation was at its maximum at 24oC (El-Wailly 1966). In Blue-winged Teal Anas discolor, Owen (1970) found a constant minimum of assimilation efficiency between -20oC and 30oC and considerably higher values at very low (-28oC) and at very high (35oC) temperatures, respectively. For Bald Eagles Haliaeetus leucocephalus, Stalmaster & Gessaman (1982, 1984) reported on a steadily decreasing energy utilisation efficiency with increasing ambient temperatures up to 10 oC, but no so above that temperature. Oystercatchers Haematopus ostralegus decreased energy assimilation efficiency with increasing ambient temperatures from 89% at 16oC to 70% at 29oC (Kersten & Piersma 1987, Klaassen et al. 1990). Garden Warblers exposed to ambient temperatures between 10 and 40oC, maintained a constant assimilation efficiency up to 30oC and increased subsequently. At 40oC, assimilation efficiency was about 60% higher than at 25oC (Bairlein 1993).
Related to ambient temperature may be the effects of water on assimilation efficiency, but the few studies available do not show a consistent pattern. In Chipping Sparrows Spizella passerina, utilisation coefficient was about 8% higher in water deprived birds than for birds on ad libitum water (Moldenhauer & Taylor 1973). The lower excretory output is seen due to the longer retention of food in the alimentary tract for re-absorption of water which results in additional assimilation of food. In contrast, water deprived Ostrich Struthius camelus reduced dry matter and energy assimilation efficiency by 50% and 35%, respectively, compared to ad libitum water supply (Withers 1983), but they also lost mass, thus the lower assimilation efficiencies may be partly due to endogenous losses.
Assimilation efficiency in chicks
Compared to adult birds, much less is known about energy and nutrient assimilation efficiencies in nestlings and chicks. Just 146 cases of 33 species of 11 taxa could be gathered for the current analysis, of which 32 cases were of domestic chicks.
Energy utilisation efficiency in young birds is influenced by age, but the patterns are not consistent. In Jackdaw Corvus monedula (Kaminski 1986) and Double-crested Cormorant Phalacrocorax auritus (Dunn 1975), energy assimilation efficiencies increased with nestling age, while it decreased with age in the European Starling (Westerterp 1973). In nestling House Sparrow, energy assimilation efficiency increased from 55.7% on day 1 to 70% on day 6-7 and later decreased to on average 67.7% on days 9 to14 (Blem 1975). Both in Snowy Owls Nyctea scandiaca and in Long-eared Owls, energy utilisation efficiency significantly decreased with increasing age (Ceska 1980, Wijnandt 1984), as it is in nestling White Ibis Eudocimus albus (Kushlan 1977). In Black-bellied Whistling Duck Dendrocygna autumnalis, assimilation efficiency slightly decreased the first 4 weeks of age to 65% in 4 week old chicks, and then increased progressively to 89% in 9 week old chicks (Cain 1976). For nestling Great Tits Parus major, Eguchi (1980) found energy assimilation efficiencies between 52 and 72%, being maximum at day 8. No significant relationship to chick age was found in Black Oystercatcher Haemantopus moquini, with assimilation efficiencies in growing chick of on average 72% and 73% in subadults, respectively (Hockey 1984). In Songthrushes Turdus philomelos, over-fed nestlings tended to have lower dry matter utilisation efficiency than the nestlings fed intermediate amount of food, and food restricted nestlings showed lowest efficiency (Konarzewski et al. 1996). It is suggested that the rate of production of nitrogen waste in food restricted nestlings was comparable to those in non-restricted ones. Hence, apparently low levels of dry matter digestibility in food restricted nestlings could be caused by high levels of urinary nitrogen compounds in their faeces, rather than in efficiency of their digestive tracts.
As with adult birds, assimilation efficiency can be affected by tannins which have detrimental effects on growth and digestive physiology of chickens. Chami et al. (1980) found reduced digestions in chickens fed soybean or corn meal with as little as 1% added tannic acid.
CONCLUSION
Irrespective of some shortcomings and pitfalls with the data, it is apparent from the current analysis that assimilation efficiency of food in birds shows considerable variation. Most of variation is due to food and its physical and chemical properties, but other factors influencing utilisation efficiencies of energy, nutrients and other food constituents must be considered in order to learn more about the adaptive significance of variable assimilation efficiency.
The mechanisms underlying the observed variation in assimilation efficiency are not clear. Digestive efficiencies are determined by complex interactions of numerous variables (Karasov & Diamond 1988). Namely gut anatomy and morphology and its functional properties are likely to play a significant role (e. g. Karasov 1990, 1996, Barton & Houston 1993b, Robbins 1993, Klasing 1998).
The ecological significance of differences in utilisation efficiencies between and within species remains open. Differences in digestive efficiency of species, irrespective of the effect of type of food, may be due to different capabilities to process and absorb food, to different physiological requirements, or they may reflect different feeding habits. A greater variance in digestive efficiencies may be characteristic of generalist feeders, whereas a smaller variance may characterize birds with narrower dietary habits. Generalist feeders forage on feeds of diverse array of quality and quantity and may show a higher degree of plasticity in their gut anatomy depending on food type than specialist feeders (e.g. Barton & Houston 1993a, 1994).
Digestive flexibility within a species may be a pre-requisite to meet digestive bottlenecks when birds are involved in extremely high energy expenditures (e.g. migratory fattening, egg-formation, moult, temperature stress). Clench & Mathias (1992) proposed a recycling hypothesis that explains how birds may digest food more completely on one occasion than on another.
Variation in assimilation efficiencies of energy and nutrients is more than simply the effect of different food. However, we are only at the beginning of a better understanding of its adaptive significance. Digestive efficiency reflects how effectively an animal exploits food resources. It is, therefore, one of the most basic parameters determining how the animal meets its energetic requirements (Weiner 1992). The nutritional content of a food item is not an accurate predictor of its nutritional value. Nutritional value is determined by the birds’ digestive efficiencies.
A better understanding of avian digestive efficiency and digestive plasticity is not only of basic interest. To know how efficiently and quickly food is processed by a bird should also be considered in conservation strategies to improve wildlife habitats. Knowing digestibility coefficients is essential for calculating the energy budget of a species and for determining how much food is required to meet daily nutritional requirements (e.g. Jorde et al. 1995). These coefficients are also useful for understanding resource partitioning by different species, e.g. geese grazing in the same area (Buchsbaum et al. 1986).
Digestive physiology is important to ecologists. A central issue is the extent to which digestive processing influences diet vs. the extent to which diet influences digestive processing (Levey & Martinez del Rio 1998). There is much to be learned in the future.
ACKNOWLEDGEMENTS
W. H. Karasov made valuable suggestions on the manuscript. Part of the study was supported by the German Research Council (DFG).
REFERENCES
Aerts, B., Esselink, P. & Helder G.J.F. 1996. Habitat selection and diet composition of Greylag Geese Anser anser and Barnacle Geese Branta leucopsis during fall and spring staging in relation to management in the tidal marshes of the Dollard. Zeitschrift für Ökologie und Naturschutz 5: 65-75.
Amat, J.A., Garcia-Criado, B. & Garcia-Ciudad, A. 1991. Food, feeding behavior and nutritional ecology of wintering greylag geese Anser anser. Ardea 79: 271-282
Bairlein, F. 1985. Efficiency of food utilisation during fat deposition in the long distance migratory Garden Warbler, Sylvia borin. Oecologia 68: 118-125
Bairlein, F. 1987. Nutritional requirements for maintenance of body weight and fat deposition in the long-distance migratory Garden Warbler, Sylvia borin (Boddaert). Comparative Biochemistry and Physiology 86A: 337-347.
Bairlein, F. 1993. Ecophysiological problems with Arctic migrants in the hot tropics. Proceeding VIII Pan-African Ornithological Congress: 571-578.
Bairlein, F. 1996. Fruit-eating in birds and ist nutritional consequences. Comparative Biochemistry and Physiology 113A: 215-224.
Bairlein, F. & Simons, D. 1995. Nutritional adaptations in migrating birds. Israel Journal of Zoology 41: 357-367.
Barton, N.W.H. & Houston, D.C. 1993a. A comparison of digestive efficiency in birds of prey. Ibis 135: 363-371.
Barton, N.W.H. & Houston, D.C. 1993b. The influence of gut morphology on digestion time in raptors. Comparative Biochemistry and Physiology 105 A: 571-578.
Barton, N.W.H. & Houston, D.C. 1994. Morphological adaptation of the digestive tract in relation to feeding ecology of raptors. Journal of Zoology, London 232: 133-150.
Bédard, J., & Gauthier, G. 1989. Comparative energy budgets of greater snow geese Chen caerlescens atlantica staging in two habitats in spring. Ardea 77: 3-20.
Bennett, D.C. & Hart, L.E. 1993. Metabolizable energy of fish when fed to captive Great Blue Herons (Ardea herodias). Canadian Journal of Zoology 71: 1767-1771.
Bernays, E. A., Cooper Driver, G. & Bilgener, M. 1989. Herbivores and plant tannins. Advances in Ecological Research 19: 263-302.
Bhatt, D. & Chandola, A. 1985. Circannual rhythm of food intake in spotted munia and its phase relationship with fattening and reproductive cycles. Journal of Comparative Physiology A 156: 429-432.
Blem, C.R. 1975. Energetics of nestling House Sparrows Passer domesticus. Comparative Biochemistry and Physiology 52 A: 305-312.
Blem, C.R. 1976. Efficiency of energy utilisation of the house sparrow Passer domesticus. Oecologia 25: 257-264.
Boudewijn, T. 1984. The role of digestibility in the selection of spring feeding sites by Brent Geese. Wildfowl 35: 97-105.
Brekke, B. & Gabrielsen, G.W. 1994. Assimilation efficiency of adult Kittiwakes and Brünnich's Guillemots fed capelin and arctic cod. Polar Biology 14: 279-284.
Brenner, F.J. 1966. Energy and nutrient requirements of the Red-winged Blackbird. Wilson Bulletin 78: 111-120.
Brooks, W.S. 1968. Comparative adaptions of the Alaskan Redpolls to the Arctic Environment. Wilson Bulletin 80: 253-280.
Brugger, K.E. 1992. Differential digestibilities of channel catfish (Ictalurus punctatus), bluegill (Lepomis macrochirus) and gizzard shad (Dorosoma cepedianum): In vitro standards for gastric digestion by seabirds. Colonial Waterbirds 15: 257-260
Brugger, K.E. 1993. Digestibility of three fish species by double-crested cormorants. Condor 95: 25-32.
Bruns, K. & Ten Thoren, B. 1990. Zugvorbereitung und Zugunruhe bei der Ringelgans Branta bernicla bernicla. Current Topics in Avian Biology: 223-229.
Bryant, D.M. & Bryant, V.M.T. 1988. Assimilation efficiency and growth of nestling insectivores. Ibis 130: 268-274.
Buchsbaum, R., Wilson, J. & Valiela, I. 1986. Digestibility of plant constituents by Canada Geese and Atlantic Brant. Ecology 67: 386-393.
Cain, B. W. 1976. Energetics of growth for black-bellied tree ducks. Condor 78: 124-128.
Castro, G., Myers, J.P. & Place, A.R. 1989a. Assimilation efficiency of Sanderlings (Calidris alba) feeding on Horseshoe Crab (Limulus polyphemus) eggs. Physiological Zoology 62: 716-731.
Castro, G., Stoyan, N. & Myers, J.P. 1989b. Assimilation efficiency in birds: a function of taxon or food type? Comparative Biochemistry and Physiology 92A: 271-278.
Ceska, V. 1980. Untersuchungen zu Nahrungsverbrauch, Nahrungsnutzung und Energiehaushalt bei Eulen. Journal für Ornithologie 121: 186-199.
Chami, D.B., Vohra, P. & Dratzer, F.H. 1980. Effect of tannin content of grain sorghums on their feeding value for growing chicks. Poultry Science 43: 30-36.
Cippolini, M.L. & Stiles, E.W. 1993. Fruit rot, antifungal defense, and palatability of fleshy fruits for frugivorous birds. Ecology 74: 751-762.
Clench, M.H. & Mathias, J.R. 1992. Intestinal transit: how can it be delayed long enough for birds to act as long-distance dispersal agents? Auk 109: 933-936.
Clifford, A.J., Smith, L.M., Creveling, R.K. Hamblin, C.L. & Clifford, C.K. 1986. Effects of dietary triglycerides on serum and liver lipids and sterol of rats. Journal of Nutrition 116: 944-956.
Daan, S., Masman, D. & Groenewold, A. 1990. Avian basal metabolic rates: their association with body compositionand energy expenditure in nature. American Journal of Physiology 259: R333-R340.
Daan, S., Masman, D., Strijkstra A.M. & Kenagy, G.J. 1991. Daily energy turnover during reproduction in birds and mammals: its relationship to basal metabolic rate. Acta XX Congressus Ornithologici: 1976-1988.
Dale, N.M., Fuller, H.L. & Pesti. G.M. 1985. Freeze drying versus oven drying of excreta in true metabolizable energy, nitrogen-corrected true metabolizable energy, and true amino acid availability bioassays. Poultry Science 64: 362-365.
Davis, E.A. 1955. Seasonal changes in the energy balance of the English Sparrow. Auk 72: 385-411.
Denbow, D.M. 1985. Food intake control in birds. Neuroscience & Biobehavioral Reviews 9: 223-243.
Diamond, A.W. & Place, A.R. 1988. Wax digestion by black-throated honeyguides (Indicator indicator). Ibis 130: 558-561.
Drent, K., Ebbinge, B. & Weijand, B. 1978/79. Balancing the energy budgets of arctic-breeding geese throughout the annual cycle: a progress report. Verhandlungen der ornithologischen Gesellschaft Bayern 23: 239-264.
Duke, G.E. & Evanson, O.A. 1972. Inhibition of gastric motility by duodenal contents in turkeys. Poultry Science 51: 1625-1636.
Duke, G.E., Reynhout, J., Tereick, A.L., Place, A.E. & Bird, D.M. 1997. Gastrointestinal morphology and motility in American Kestrels receiving high or low fat diets. Condor 99: 123-131.
Dunn, E.H. 1975. Caloric intake of nestling double-crested cormorants. Auk 92: 553-565.
Dunning, J.B. Jr. 1993. CRC Handbook of Avian Body Masses. Boca Raton; CRC Press: 371pp.
Eggel, W. 1991. Ernährungsphysiologische Adaptationen extremer Nahrungsspezialisten, der Fruchttauben (Aves, Columbidae). Thesis, University of Zurich, Zürich, Switzerland.
Eguchi, K. 1980. The feeding ecology of the nestling Great Tit, Parus major minor, in the temperate ever-green broadleaved forest. 1 Food consumption and maintenance cost. Journal of Yamashina Institute for Ornithology 12: 68-78.
El-Wailly, A.J. 1966. Energy requirements for egg-laying and incubation in the Zebra Finch, Taeniopygia castanotis. Condor 68: 582-594.
Fry, H.H., Ferguson-Lees, I.J. & Dowsett, R.J. 1972. Flight muscle hyperthropy and ecophysiological variation of yellow wagtail Motacilla flava races at Lake Chad. Journla of Zoology London 167: 293-306.
Furness, R.W. 1978. Energy requirements of seabird communities: a bioenergetics model. Journal of Animal Ecology 47: 39-53
Gasaway, W.C., White, R.G. & Holleman, D.F. 1976. Digestion of dry matter and absorption of water in the intestine and cecum of Rock Ptarmigan. Condor 78: 77-84.
Gifford, C.F. & Odum, E. 1965. Bioenergetics of lipid deposition in the bobolink, a trans-equatorial migrant. Condor 67: 383-403.
Grajal, A. 1995. Digestive efficiency of the Hoatzin, Opisthocomus hoazin: a folivorous bird with foregut fermentation. Ibis 137: 383-388.
Guglielmo, C. & Karasov, W.H. 1993. Endogenous mass and energy losses in Ruffed Grouse. Auk 110: 386-390.
Guglielmo, C.G. & Karasov, W.H. 1995. Nutritional quality of winter browse for Ruffed Grouse. Journal of Wildlife Management 59: 427-436.
Guglielmo, C.G., Karasov, W.H. & Jakubas, W.J. 1996. Nutritional costs of a plant secondary metabolite explain selective foraging by Ruffed Grouse. Ecology 77: 1103-1115.
Herd, R.M. & Dawson, T.J. 1984. Fiber digestion in the Emu, Domaius novahollandiae: a large bird with a simple gut and high rates of passage. Physiological Zoology 57: 70-84
Hirano, S., Senda, H., Yamamoto, Y. & Watanabe, A. 1984. Several novel attemots for the use of the potential functions of chitin and chitosan. In: Zikakis, J. P. (ed.): Chitin, chitosan and related enzymes. Academic Press, Orlando: 77-95.
Hockey, P.A.R. 1984. Growth energetics of the African Black Oystercatcher, Haematopus moquini. Ardea 72: 111-117
Hoffmann, R. & Prinzinger, R. 1984. Torpor und Nahrungsausnutzung bei 4 Mausvogelarten (Coliiformes). Journal für Ornithologie 125: 225-237.
Hume, I.D. & Biebach, H. 1996. Digestive tract function in the long-distance migratory Garden Warbler, Sylvia borin. Journal of Comparative Physiology B 166: 388-395.
Hupp, J.W., White, R.G., Sedinger , J.S. & Robertson, D.G. 1996. Forage digestibility and intake by lesser snow geese: effects of dominance and resource heterogenity. Oecologia 108: 232-240.
Iason, G.R. & Murray, A.H. 1996. The energy costs of ingestion of naturally occurring nontannin plant phenolics by sheep. Physiological Zoology 69: 532-546
Jackson, S., & Place, A.R. 1990. Gastrointestinal transit and lipid assimilation efficiency in three species of sub-Antarctic seabird. Journal of Experimental Zoology 255: 141-154
Jackson, S., Place, A.R. & Seiderer, L.J. 1992. Chitin digestion and assimilation by seabirds. Auk 109: 758-770
Jackson, S., Nicolson, S.W. & van Wyk, B.-E. 1998. Apparent absorption efficiencies of nectar sugars in the Cape Sugarbird, with a comparison of methods. Physiological Zoology 71: 106-115.
Jakubas, W.J., Karasov, W.H. & Guglielmo, C.G. 1993. Coniferyl benzoate in Quaking Aspen (Populus tremuloides): its effect on energy and nitrogen digestion and retention in Ruffed Grouse (Bonasa umbellus). Physiolocial Zoology 66: 580-601.
Jeuniaux, C. & Cornelius, C. 1978. Distribution and activity of chitinolytic enzymes in the digestive tract of birds and mammals. In: Muzzarelli, R. A. A. & Pariser, E. R. (eds): Proceedings of the First International Conference on Chitin/Chitosan. Massachusetts Institute of Technology, Cambridge, Massachusetts: 542-549.
Johnson, W.C., Thomas, L. & Adkisson, C.S. 1993. Dietary circumvention of acorn tannins by blue jays. Oecologia 94: 159-164.
Jorde, D.G., Haramis, G.M., Bunck, C.M. & Pendleton, G.W. 1995. Effects of diet on rate of body mass gain by wintering Canvasback. Journal of Wildlife Management 59: 31-39.
Kaminski, P. 1986. Bioenergetische Untersuchungen zur Jugendentwicklung der Dohle (Corvus monedula). Journal für Ornithologie 127: 315-329.
Karasov, W.H. 1990. Digestion in birds: Chemical and physiological determinants and ecological implications. In: Morrison, M.L., Ralph, C.J., Verner, J. & Jehl, R.R. (eds) Avian Foraging: Theory, methodology, and applications. Los Angeles, California; Cooper Ornithological Society: 391-415.
Karasov, W.H. 1996. Digestive plasticity in avian energetics and feeding ecology. In: Carey, C. (ed.) Avian energetics and nutritional ecology. New York; Chapman & Hall: 61-84.
Karasov, W.H. & Diamond, J.M. 1988. Interplay between physiology and ecology in digestion. BioScience 38: 602-611
Karasov, W.H. & Levey, D.J. 1990. Digestive system tradeoffs and adaptations of passerine frugivorous birds. Physiological Zoology 63: 1248-1270.
Karasov, W.H. & Cork, S.J. 1996. Test of a reactor-based digetion optimization model for nectar-eating Rainbow Lorkeets. Physiological Zoology 69: 117-138.
Kendeigh, S.C. 1949. Effects of temperature and season on energy resources of the English Sparrow. Auk 66: 113-127.
Kendeigh, S.C. 1969. Energy responses of birds to their thermal environments. The Wilson Bulletin 81: 441-449.
Kendeigh, S.C. 1972. Monthly variations in the energy budget of the House Sparrow throughout the year. In: Kendeigh, S. C. & Pinowski, J. (eds) Productivity, population dynamics and systematics of granivorous birds. Warszawa: 17-43.
Kendeigh, S.C., Dolnik, V.R. & Gavrilov, V.M. 1977. Avian energetics. In: Pinowski, I. & Kendeigh, S.C. (eds) Granivorous birds in ecosystems. Cambridge; Cambridge UP: 127-204.
Kersten, M. & Piersma, T. 1987. High levels of energy expenditure in shorebirds: metabolic adaptations to an energetically expensive way of life. Ardea 75: 175-187.
Kirkwood, J.K. 1979. The partition of food energy for existence in the Kestrel Falco sparverius and the Barn Owl Tyto alba. Comparative Biochemistry and Physiology 63 A: 495-498.
Klaassen, M., Kersten, M. & Ens, B.J. 1990. Energetic requirements for maintenance and premigratory body mass gain of waders wintering in Africa. Ardea 78: 209-220.
Klasing, K.C. 1998. Comparative Avian Nutrition. CAB International, Oxon.
Koenig, W.D. 1991. The effects of tannins and lipids on digestion of acorns by Acorn Woodpeckers. Auk 108: 79-88.
Konarzewski, M., Kowalczyk, J., Swierubska, T. & Lewonczuk, B. 1996. Effect of short-term feed restriction, realimentation and overfeeding on growth of Song Thrush (Turdus philomelos) nestlings. Functional Ecology 10: 97-105.
Kontogiannis, J.E. 1968. Effect of temperature and exercise on energy intake and body weight of the White-throated Sparrow (Zonotrichia albicollis). Physiological Zoology 41: 54-64.
Krebs, R. & Avery, M.I. 1984. Chick growth and prey quality in the European Bee-eater (Merops apiaster). Oecologia 64: 363-368.
Kushland, J.A. 1977. Growth energetics of the White Ibis. Condor 79: 31-36.
Lane, S. & Hassall, M. 1996. Estimation of apparent metabolizability in herbivorous wildfowl with plant pigments. Journal of Wildlife Management 60: 910-916.
Lauterbach, S. & Prinzinger, R. 1994. Ernährungsphysiologie des Blaunackenmausvogels (Urocolius macrourus pulcher). Journal für Ornithologie 135: 577-586.
Levey, D.J., & Karasov, W.H. 1989. Digestive responses of temperate birds switched to fruit or insect diets. Auk 106: 675-686
Levey, D.J., & Martínez del Rio, C. 1998. Rapid modulation of retention time in a fruit-eating bird: Ecological implications and test of a model. Physiological Zoology: in press
Lindström, A. & Kvist, A. 1995. Maximum energy intake rate is proportional to basal metabolic rate in passerine birds. Proceedings Royal Society of London B 261: 337-343.
Martin, E.W. 1968. The effects of dietary protein on the energy and nitrogen balance of the Tree-Sparrow (Spizella arborea arborea). Physiological Zoology 41: 313-331.
Martinez del Rio, C. 1990. Dietary, phylogenetic, and ecological correlates of intestinal sucrase and maltase activity in birds. Physiological Zoology 63: 987-1011.
Martinez del Rio, C., Stevens, B.R., Daneke, D.E. & Andreadis, P.T. 1988. Physiological correlates of preference and aversion for sugars in three species of birds. Physiological Zoology 61: 222-229.
Martinez del Rio, C., Karasov, W.H. & Levey, D.J. 1989. Physiological basis and ecological consequences of sugar preference in cedar waxwings. Auk 106: 64-71.
Martinez del Rio, C., Baker, H.G. & Baker, I. 1992. Ecological and evolutionary implications of digestive processes: Bird preferences and the sugar constituents of floral nectar and fruit pulp. Experientia 48: 544-551.
Masman, D. 1986. The annual cycle of the kestrel Falco tinnunculus. Thesis, University of Groningen, Groningen, The Netherlands.
Mateos, G.G. & Sell, J.L. 1980. Influence of carbohydrate and supplemental fat source on the metabolizable energy of the diet. Poultry Science 59: 2129-2135.
Mateos, G.G. & Sell, J.L. 1981. Metabolizable energy of supplemental fat as related to dietary fat level and methods of estimation. Poultry Science 60: 1509-1515.
McWilliams, S.R. & Karasov, W.H. 1998. Test of a digestion optimization model: effects of costs of feeding on digestive parameters. Physiological Zoology 71: 168-178.
McNab, J.M. & Shannon, D.W.F. 1974. The nutritional value of barley, maize, oats, and wheat for poultry. British Poultry Science 15: 561-567.
Meienberger, C. & Ziswiler, V. 1990. Die Artabhängigkeit der Umsatzquotienten nahverwandter Vögel - ein mathematisches Problem? Journal für Ornithologie 131: 267-277.
Merkel, F.W. 1958. Untersuchungen über tages- und jahresperiodische Änderungen im Energiehaushalt gekäfigter Zugvögel. Zeitschrift für vergleichende Physiologie 41: 154-178.
Mlingwa, C. 1997. Comparative feeding ecology of coexisting bulbuls in coastal Tanzania. PhD Thesis, University of Oldenburg, Oldenburg, Germany.
Moldenhauer, R.R. & Taylor, P.G. 1973. Energy intake by hydropenic Chipping Sparrows (Spizella passerina passerina) maintained on different diets. Condor 75: 439-445.
Mole, S. & Waterman, P.G. 1985. Stimulatory effects of tannins and cholic acid on tryptic hydrolysis of proteins: ecological implications. Journal of Chemical Ecology 11: 1323-1332.
Mole, S. & Waterman, P.G. 1987. Tannins as antifeedents to mammalian herbivores - still an open question? In: Waller, G. R. (ed.) Allelochemicals: Role in agriculture and forestry. Washington; A. C. S.: 572-587.
Mortensen, A. & Blix, A.S. 1989. Seasonal changes in energy intake, energy expenditure, and digestibility in captive Svalbard Rock Ptarmigan and Norwegian Willow Ptarmigan. Ornis Scandinavica 20: 22-28.
Moss, R. 1977. The digestion of heather by Red Grouse during the spring. Condor 79: 471-477.
Moss, R., Gardarsson, A., Olafsson, G. & Brown, D. 1974. The in vitro digestibility of Ptarmigan Lagopus mutus foods in relation to their chemical composition. Ornis Scandinavica 5: 5-11.
Moss, R. & Parkinson, J.A. 1972. The digestion of heather (Calluna vulgaris) by Red Grouse (Lagopus lagopus scoticus). British Journal of Nutrition 27: 285-298.
Moss, R. & Trenholm, I.B. 1987. Food intake, digestibility and gut size in Red Grouse. British Poultry Science 28: 81-89.
Murphy, M.E. 1993. The protein requirement for maintenance in the White-crowned Sparrow, Zonotrichia leucophrys gambelii. Canadian Journal of Zoology 71: 2111-2120.
Nelson, J.T., Slack, R.D. & Gee, G.F. 1996. Nutritional value of winter foods for Whooping Cranes. Wilson Bulletin 108: 728-739.
Novoa, F.F., Veloso, C., López-Calleja, M.V. & Bozinovic, F. 1996. Seasonal changes in diet, digestive morphology and digestive efficiency in the Rufous-collared Sparrow (Zonotrichia capensis) in central Chile. Condor 98: 873-876.
Obst, B.S. 1986. Wax digestion in Wilson's Storm-Petrel. Wilson Bulletin 98: 189-195.
Parrish, I.W. & Martin, E.W. 1977. The effect of dietary lysine level on the energy and nitrogen balance of the dark-eyed junco. Condor 79: 24-30.
Peoples, A.D., Lochmiller, R.L., Leslie, D.M.J., Boren, J.C. & Engle, D.M. 1994. Essential amino acids in northern bobwhite foods. Journal of Wildlife Management 58: 167-175.
Peterson, J. & Wunder, B.A. 1997. Food sorting by Lollared Lemmings (Dicrostonyx groenlandicus) and Prairie Voles (Microtus ochrogaster): A cautionary note for digestibility studies. Comparative Biochemistry and Physiology 116A: 119-124.
Place, A.R. & Stiles, E.W. 1992. Living off the wax of the land: Bayberries and Yellow-rumped Warblers. Auk 109: 334-345.
Prop, J., & Vulink, T. 1992. Digestion by Barnacle Geese in the annual cycle: The interplay between retention time and food quality. Functional Ecology 6: 180-189
Pusztai, A., Grant, G., Duguid, T., Brown, D.S., Peumans, W.J., Van Damme, E.J.M. & Bardocz, S. 1995. Inhibition of starch digestion by a -amylase inhibitor reduces the efficiency of utilisation of dietary proteins and lipids and retards the growth of rats. Journal of Nutrition 125: 1554-1562.
Renner, R. & Hill, W.F. 1961. Utilisation of fatty acids by chicken. Journal of Nutrition 74: 259-264.
Ricklefs, R.E., Konarzewski, M. & Daan, S. 1996. The relationship between basal metabolic rate and daily energy expenditure in birds and mammals. American Naturalist 147: 1047-1071.
Robbins, C.T. 1993. Wildlife feeding and nutrition. San Diego, Academic Press: 352pp.
Roby, D.D., Place, A.R. & Ricklefs, R.E. (1986). Assimilation and deposition of dietary wax esters in planktivorous seabirds. Journal of Experimental Zoology 238: 29-41.
Sedinger, J.S., White, R.G., & Hupp, J. 1995. Metabolizability and partitioning of energy and protein in green plants by yearling lesser snow geese. Condor 97: 116-122
Seibert, H.C. 1949. Differences between migrant and non-migrant birds in food and water uptake at various temperatures and photoperiods. Auk 66: 128-153.
Seldal, T., Andersen, K.-J. & Högstedt, G. 1994. Grazing-induced proteinase inhibitors: a possible cause for lemming population cycles. Oikos 70: 3-11.
Servello, F.A. & Kirkpatrick, R.L. 1989. Nutritional value of acorns for Ruffed Grouse. Journal of Wildlife Management 53: 26-29.
Sibbald, I.R. 1980. Metabolizable energy in poultry nutrition. BioScience 30: 736-741.
Sibly, R.M. 1981. Strategies of digestion and defecation. In Townsend, C. R. and Calow, P. (eds): Physiological Ecology, Sunderland, MA, pp. 109-139.
Simons, D. 1993. Die adaptive Bedeutung saisonaler Frugivorie für die zugzeitliche Depotfettbildung bei der Gartengrasmücke Sylvia borin. PhD Thesis, University of Cologne, Cologne, Germany.
Stalmaster, M.V. & Gessaman, J.A. 1982. Food consumption and energy requirements of captive bald eagles. Journal of Wildlife Management 46: 646-654.
Stalmaster, M.V. & Gessaman, J.A. 1984. Ecological energetics and foraging behavior of overwintering Bald Eagles. Ecological Monographs 54: 407-428.
Sues, M. 1974. Digestibility and metabolizable energy content of beef tallow for laying hens. Proceedings 25th World Poultry Congress, New Orleans: 367-369.
Weiner, J. 1992. Physiological limits to sustainable energy budgets in birds and mammals: ecological implications. Trends in Ecology & Evolution 7: 384-388
Weiser, J.I., Porth, A., Mertens, D. & Karasov, W.H. 1997. Digestion of chitin by northern Bobwhites and American Robins. Condor 99: 554-556.
Welsch, C.A., Lachance, R.A. & Wasserman, B.P. 1989. Dietary phenolic compounds: inhibition of Na-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. Journal of Nutrition 119: 1698-1704.
West, G.G. 1960. Seasonal variation in the energy balance of the tree sparrow in relation to migration. Auk 77: 306-329.
West, G.C. 1968. Bioenergetics of captive willow ptarmigan under natural conditions. Ecology 49: 1035-1045.
Westerterp, K. 1973. The energy budget of the nestling Starling Sturnus vulgaris, a field study. Ardea 61: 137-158.
Wijnandts, H. 1984. Ecological energetics of the Long-eared Owl Asio otus. Ardea 72: 1-92.
Willson, M.F. & Harmeson, J.C. 1973. Seed preferences and digestive efficiency of Cardinals and Song Sparrows. Condor 75: 225-234.
Withers, P.C. 1983. Energy, water and solute balance of the Ostrich, Struthio camelus. Physiological Zoology 56: 568-579
Zach, R. & Falls, J. 1978. Prey selection by captive ovenbirds (Aves: Parulidae). Journal of Animal Ecology 47: 929-943.
Zbinden, N. 1980. Zur Verdaulichkeit und umsetzbaren Energie von Tetraoniden-Winternahrung und zum Erhaltungsbedarf des Birkhuhns (Tetrix tetrix) in Gefangenschaft mit Hinweisen auf Verdauungsversuche. Vogelwelt 101: 1-18.
Zimmerman, J.L. 1968. Digestive efficiency and premigratory obesity in the dickcissel. Auk 82: 278-279.
Zwarts, L. & Blomert, A.-M. 1990. Selectivity of Whimbrels feeding on fiddler crabs explained by component specific digestibilities. Ardea 78: 193-208.
Table 1. Assimilation efficiency (metabolic energy coefficient MEC) in birds (mean ± s.e.) grouped by taxon and food type. n.s.= not significant; figure in brackets = sample size. Analysis of variance (ANOVA) was carried out to seek for differences among and within taxa and food type. Analysis of covariance (ANCOVA) was used to account for the effect of food energy content.

Table 2. Assimilation efficiency (metabolic energy coefficient MEC) in birds (mean ± s.e.) feeding on animal prey grouped by prey type.
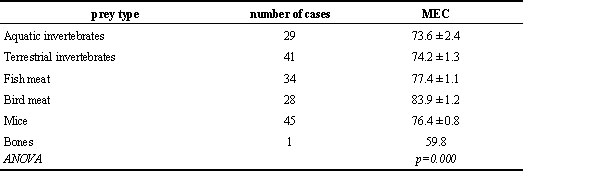
Table 3. Correlation of energy assimilation efficiency and daily energy expenditure in birds. n.s. = not significant. Only taxa with more than one species and more than 10 cases are considered.
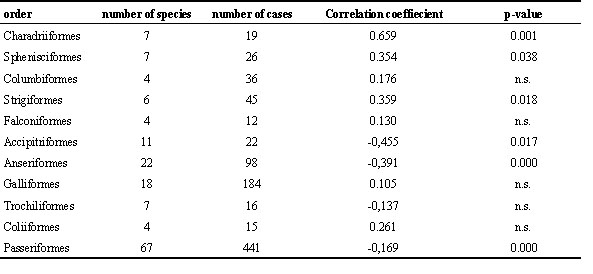
Table 4. Correlation of energy assimilation efficiency in birds with food composition. 1. line: correlation coefficient; 2. line: p-values (n.s. = not significant); 3. line: sample size.
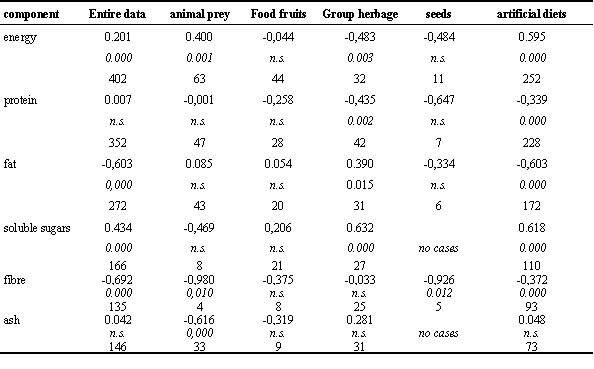
Table 5. Protein, fat, sugar and crude fibre assimilation coefficients in birds grouped by food type.
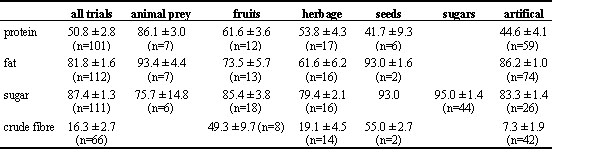
Table 6. Analysis of variance of the effect of taxon and food type on nutrient and fibre assimilation efficiency in birds.

Table 7. Dry matter assimilation efficiency in Garden Warblers Sylvia borin according to migratory fattening.
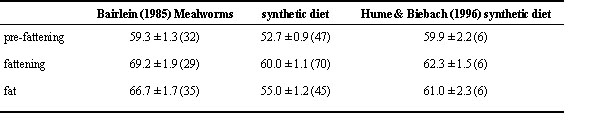
Fig. 1. Frequency distribution of energy assimilation efficiencies in birds according to major food type. Figures in brackets: number of cases.
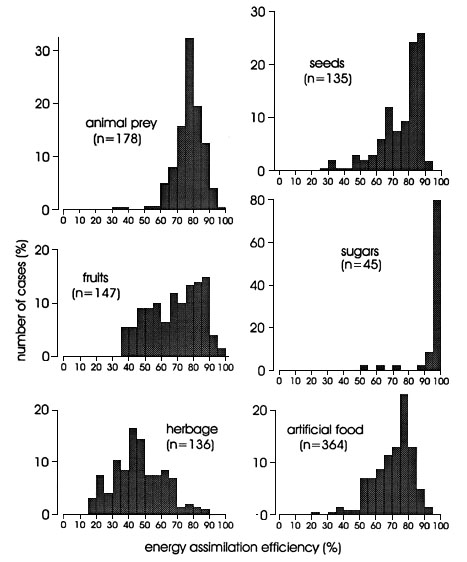
Fig. 2. Frequency distribution of protein, fat, sugar, and crude fibre assimilation efficiencies in birds. Figures in brackets: number of cases.